Abstract
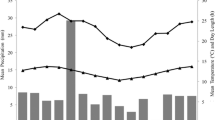
Cathemerality consists of discrete periods of activity during both the day and night. Though uncommon within Primates, cathemerality is prevalent in some lemur genera, such as Eulemur, Hapalemur, and Prolemur. Several researchers have also reported nighttime activity in Lemur catta, yet these lemurs are generally considered “strictly diurnal”. We used behavioral observations and camera traps to examine cathemerality of L. catta at the Tsimanampetsotsa National Park, Madagascar. Nighttime activity occurred throughout the study period (September 2010–April 2011), and correlated with warm overnight temperatures but not daytime temperatures. Animals spent 25 % of their daytime active behaviors on the ground, but appeared to avoid the ground at night, with only 5 % of their time on the ground. Furthermore, at night, animals spent the majority of their active time feeding (53 % nighttime, 43 % daytime). These findings imply that both thermoregulation and diet play a role in the adaptive significance of cathemerality. Additionally, predator avoidance may have influenced cathemerality here, in that L. catta may limit nighttime activity as a result of predation threat by forest cats (Felis sp.) or fossa (Cryptoprocta ferox). Further data are needed on cathemeral lemurs generally, but particularly in L. catta if we are to fully understand the evolutionary mechanisms of cathemerality in the Lemuridae.




Similar content being viewed by others
References
Altmann J (1974) Observational study of behaviour: sampling methods. Behaviour 49:227–267
Andrews J, Birkinshaw CR (1998) A comparison between the daytime and night-time diet, activity and feeding height of the black lemur, Eulemur macaco (Primates: Lemuridae), in Lokobe forest, Madagascar. Folia Primatol 69(1):175–182
Andriatsimietry R, Goodman SM, Razafimahatratra E, Jeglinski JWE, Marquard M, Ganzhorn JU (2009) Seasonal variation in the diet of Galidictis grandidieri Wozencraft, 1986 (Carnivora: Eupleridae) in a sub-arid zone of extreme south-western Madagascar. J Zool 279(4):410–415. doi:10.1111/j.1469-7998.2009.00633.x
Charles-Dominique P (1975) Nocturnality and diurnality: an ecological interpretation of these two modes of life by an analysis of higher vertebrate fauna in tropical ecosystem. In: Luckett WP, Szalay FS (eds) Phylogeny of the primates: a multidisciplinary approach. Plenum, New York, pp 69–88
Colquhoun I (2006) Predation and cathemerality: comparing the impact of predators on the activity patterns of lemurids and ceboids. Folia Primatol 77:143–163. doi:10.1159/000089701
Curtis DJ (2006) Cathemerality in lemurs. In: Gould L, Sauther ML (eds) Lemurs: ecology and adaptation. Springer, Berlin, pp 133–157. doi:10.1159/000089703
Curtis DJ, Donati G (2013) Is temporal plasticity in lemurs a strategy for dealing with unpredictable or predictable seasonal environments? In: Masters J, Gamba M, Genin F (eds) Leaping ahead. Springer, New York, pp 41–48. doi:10.1007/978-1-4614-4511-1_5
Curtis D, Rasmussen M (2002) Cathemerality in lemurs. Evol Anthropol 11:83–86. doi:10.1002/evan.10064
Curtis D, Rasmussen M (2006) The evolution of cathemerality in primates and other mammals: a comparative and chronoecological approach. Folia Primatol 77(1–2):178–193. doi:10.1159/000089703
Curtis D, Zaramody A, Martin R (1999) Cathemerality in the mongoose lemur, Eulemur mongoz. Am J Primatol 47(4):279–298. doi:10.1002/(SICI)1098-2345(1999)47:4<279::AID-AJP2>3.0.CO;2-U
Daniels HL (1984) Oxygen consumption in Lemur fulvus, deviation from the ideal model. J Mammal 65(4):584–592
Dewar RE, Richard AF (2007) Evolution of the hypervariable environment of Madagascar. Proc Natl Acad Sci USA 104(34):13723–13727. doi:10.1073/pnas.0704346104
Donati G, Borgognini-Tarli S (2006) Influence of abiotic factors on cathemeral activity: the case of Eulemur fulvus collaris in the littoral forest of Madagascar. Folia Primatol 77(1–2):104–122. doi:10.1159/000089698
Donati G, Lunardini A, Kappeler PM (1999) Cathemeral activity of red-fronted brown lemurs (Eulemur fulvus rufus) in the Kirindy Forest/CFPF. In: Rakotosamimanana B, Rasamimanana H, Ganzhorn JU, Goodman SM (eds) New directions in lemur studies. Kluwer Acad/Plenum, New York, pp 119–137
Donati G, Baldi N, Morelli V, Ganzhorn JU, Borgognini-Tarli SM (2009) Proximate and ultimate determinants of cathemeral activity in brown lemurs. Anim Behav 77(2):317–325. doi:101016/janbehav200809033
Donati G, Ricci E, Baldi N, Morelli V, Borgognini-Tarli SM (2011) Behavioral thermoregulation in a gregarious lemur, Eulemur collaris: effects of climatic and dietary-related factors. Am J Phys Anthropol 144(3):355–364. doi:10.1002/ajpa.21415
Donati G, Santini L, Razafindramanana J, Boitani L, Borgognini-Tarli S (2013) (Un-)expected nocturnal activity in “diurnal” Lemur catta supports cathemerality as one of the key adaptations of the lemurid radiation. Am J Phys Anthropol 150(1):99–106. doi:10.1002/ajpa.22180
Donque G (1975) Contribution géographique à l’étude du climat de Madagascar. Nouvelle imprimerie des arts graphiques, Antananarive
Ellwanger N, Gould L (2011) Variations in behavioural patterns between Lemur catta groups living in different forest types: implications for conservation. Endanger Species Res 14:259–270. doi:10.3354/esr00362
Engqvist A, Richard A (1991) Diet as a possible determinant of cathemeral activity patterns in primates. Folia Primatol 57:169–172. doi:10.1159/000156581
Enright J (1970) Ecological aspects of endogenous rhythmicity. Annu Rev Ecol Syst 1:221–228
Fernandez-Duque E (2003) Influences of moonlight, ambient temperature, and food availability on the diurnal and nocturnal activity of owl monkeys (Aotus azarai). Behav Ecol Sociobiol 54(5):431–440. doi:10.1007/s00265-003-0637-9
Ganzhorn JU, Wright PC, Ratsimbazafy HJ (1999) Primate communities: Madagascar. In: Fleagle JG, Janson CH, Reed K (eds) Primate communities. Cambridge University Press, Cambridge, pp 75–89
Ganzhorn JU, Rakotondranary SJ, Ratovonamana YR (2007) Habitat description and phenology. In Setchell JM, Curtis DJ (eds) Field and laboratory methods in primatology: a practical guide. Cambridge University Press, London, pp 51–68
Gese EM (2001) Monitoring of terrestrial carnivore populations. USDA National Wildlife Research Center. 576. http://digitalcommons.unl.edu/icwdm_usdanwrc/576
Goering HK, Van Soest PJ (1970) Forage fiber analysis (apparatus, reagents, procedures and some applications). In: Agriculture handbook no. 379. Agriculture Research Service, United States Department of Agriculture, Washington
Golden C (2009) Bushmeat hunting and use in the Makira Forest, north-eastern Madagascar: a conservation and livelihoods issue. Oryx 43(3):386–393
Gould L (2006) Lemur catta ecology: what we know and what we need to know. In: Gould L, Sauther M (eds) Lemurs: ecology and evolution. Springer, Berlin, pp 257–276
Halle S (2006) Polyphasic activity patterns in small mammals. Folia Primatol 77(1–2):15–26. doi:10.1159/000089693
Hawkins CE (2003) Cryptoprocta ferox, fossa, fosa. In: Goodman SM, Benstead JP (eds) The natural history of Madagascar. University of Chicago Press, Chicago, pp 1360–1363
Hoffmann HU, Dillenberger V, Thouvenin C (1992) Vari. In: Ceska V, Hoffmann HU, Winkelsträter KH (eds) Lemuren im zoo. Paul Parey, Berlin, pp 175–203
Jolly A (1966) Lemur behavior: a Madagascar field study. Chicago University Press, Chicago
Karpanty S (2006) Direct and indirect impacts of raptor predation on lemurs in southeastern Madagascar. Int J Primatol 27(1):239–261. doi:10.1007/s10764-005-9008-x
LaFleur M (2012) Ecology of ring-tailed lemurs (Lemur catta) at the Tsimanampetsotsa National Park, Madagascar: implications for female dominance and the evolution of lemur traits. Ph.D. dissertation, University of Colorado at Boulder, USA. UMI Dissertations Publishing, document ID 1017548854
Macdonald DW, Mace GM, Barreto GR (1999) The effects of predators on fragmented prey populations: a case study for the conservation of endangered prey. J Zool 247:487–506
Mutschler T (1999) Folivory in a small-bodied lemur: The nutrition of the Alaotran gentle lemur (Hapalemur griseus alaotrensis). In: Rakotosamimanana M, Rasamimanana H, Ganzhorn JU, Goodman SM (eds) New directions in Lemur studies. Kluwer Academic/Plenum Publishers, New York, USA. pp 221–239
Mutschler T (2002) Alaotran gentle lemur: some aspects of its behavioral ecology. Evol Anthropol 11:101–104
Overdorff D (1993) Similarities, differences, and seasonal patterns in the diets of Eulemur rubriventer and Eulemur fulvus rufus in the Ranomafana National Park, Madagascar. Int J Primatol 14(5):721–753. doi:10.1007/BF02192188
Overdorff DJ, Rasmussen MA (1995) Determinants of nighttime activity in ‘‘diurnal’’ lemurid primates. In: Alterman L, Doyle GA, Izard MK (eds) Creatures of the dark. Plenum Press, New York, pp 61–74
Parga JA (2011) Nocturnal ranging by a diurnal primate: are ring-tailed lemurs (Lemur catta) cathemeral? Primates 52(3):201–205. doi:10.1007/s10329-011-0257-3
Rasmussen MA (1999) Ecological influences on activity cycle in two cathemeral primates, Eulemur mongoz (mongoose lemur) and Eulemur fulvus fulvus (common brown lemur). PhD dissertation, Duke University, Durham
Sauther M (1989) Antipredator behavior in troops of free ranging Lemur catta at Beza Mahafaly special reserve, Madagascar. Int J Primatol 10(6):595–606
Sauther ML, Cuozzo FP (2008) Somatic variation in living, wild ring-tailed lemurs (Lemur catta). Folia Primatol 79:55–78. doi:10.1159/000108589
Sauther ML, Cuozzo FP, Sussman RW (1999) An analysis of the dentition of a living wild population of ring-tailed lemurs (Lemur catta). Am J Phys Anthropol 28:241
Sauther ML, Cuozzo FP, Jacky Youssouf IA, Ness J, LaFleur M, Larsen RS, Tkach V (2011) A season of death: patterns of predation on wild lemurs at Beza Mahafaly Reserve, Madagascar using multiple methods of assessment. Am J Phys Anthropol 141:262
Sussman RW (1991) Demography and social organization of free-ranging Lemur catta in the Beza Mahafaly Reserve, Madagascar. Am J Phys Anthropol 84:43–58
Tan C (1999) Group composition, home range size, and diet of three sympatric bamboo lemur species (genus Hapalemur) in Ranomafana National Park, Madagascar. Int J Primatol 20(4):547–566
Tarnaud L (2006) Cathemerality in the Mayonette brown lemur (Eulemur fulvus): seasonality and food quality. Folia Primatol 77:166–177
Tattersall I (1982) The primates of Madagascar. Columbia University Press, New York
Tattersall I, Sussman RW (1998) “Little brown lemurs” of northern Madagascar. Folia Primatol 69:379–388. doi:10.1159/000052726
Terborgh J, Janson CH (1986) The socioecology of primate groups. Annu Rev Ecol Syst 17:111–135
Traina A (2001) Activity pattern and feeding behavior of ring-tailed lemurs (Lemur catta) at Berenty Reserve in Madagascar during the day and night. Folia Primatol 72:188
United States Naval Observatory Astronomical Applications Department (2011). http://aa.usno.navy.mil/ed
van Schaik C, Griffiths M (1996) Activity periods of Indonesian rain forest mammals. Biotropica 28(1):105–112
van Schaik CP, Kappeler PM (1996) The social systems of gregarious lemurs: lack of convergence with anthropoids due to evolutionary disequilibrium? Ethology 102:915–941
van Soest PJ (1994) Nutritional ecology of the ruminant. Cornell University Press, Ithaca
Wright P (1989) The nocturnal primate niche in the New World. J Hum Evol 18(7):635–658. doi:10.1016/0047-2484(89)90098-5
Wright PC (1999) Lemur traits and Madagascar ecology: coping with an island environment. Yearb Phys Anthropol 42:31–72. doi:101002/(SICI)1096-8644(1999)110:29+<31:AID-AJPA3>30CO;2-0
Acknowledgments
The authors thank the government of Madagascar, Madagascar National Parks and the University of Toliara, Madagascar, for granting us permission to work at Tsimanampetsotsa National Park. We are grateful for the excellent research assistance provided by Megan Hoopes and Bronwyn McNeil. We thank the Beza Mahafaly animal darting team (Enafa Efitroaromy, Edidy Ellis, and Elahavelo) and the Tsimanampetsotsa National Park ecological monitoring team (Razanajafy Olivier, Lauren, Stephan), and local experts Fiti and Francisco. We are extremely appreciative to the Lanto, Bakira Ravorona, and their families for their facilitation, assistance, and friendship. We thank the University of Hamburg and Joerg Ganzhorn for the plant food nutritional analyses. We also thank Chia Tan and two anonymous additional reviewers for their feedback, which improved this manuscript. Last, we thank the lemurs of Tsimanampetsotsa, without whom this research would have not been possible. We, the authors, confirm that there is no conflict of interest that has influenced our objectivity. Grant sponsorship: NSF DDIG 1028708, NSERC PGS 296264, National Geographic Society Committee for Research and Exploration Grant 88011, and American Society of Primatologists 2009 Small Research Grant awarded to MML. NSF BCS 0922645, ND EPSCoR, University of North Dakota Faculty Seed Money Council Award, University of Colorado (IGP, CRCW), International Primatological Society, Primate Conservation Inc., American Society of Primatologists, to MLS and/or FPC. All data presented in this manuscript complied with the protocols approved by the Institutional Animal Care and Use Committee at the University of Colorado Boulder (Protocol number 1002.09), and the ethical standards of treatment as laid down by the Primate Society of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
LaFleur, M., Sauther, M., Cuozzo, F. et al. Cathemerality in wild ring-tailed lemurs (Lemur catta) in the spiny forest of Tsimanampetsotsa National Park: camera trap data and preliminary behavioral observations. Primates 55, 207–217 (2014). https://doi.org/10.1007/s10329-013-0391-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-013-0391-1