Abstract
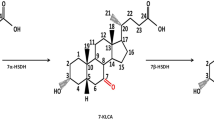
Bear bile powder is a precious medicinal material. It is characterized by high content of tauroursodeoxycholic acid (TUDCA) at a ratio of 1.0–1.5 to taurochenodeoxycholic acid (TCDCA). Here, we reported the biotransformation of tauroursodeoxycholic acid (TUDCA) through Escherichia coli engineered with a two-step mimic biosynthetic pathway of TUDCA from taurochenodeoxycholic acid (TCDCA). Two 7α-hydroxysteroid dehydrogenase (7α-HSDH) and two 7β-hydroxysteroid dehydrogenase (7β-HSDH) genes (named as α1, α2, β1, and β2) were selected and synthesized to create four pathway variants using ePathBrick. All could convert TCDCA to TUDCA and the one harboring α1 and β2 (pα1β2) showed the strongest capability. Utilizing the oxidative and reductive properties of 7α- and 7β-HSDH, an ideal balance between TUDCA and TCDCA was established by optimizing the fermentation conditions. By applying the optimal condition, E. coli containing pα1β2 (BL-pα1β2) produced up to 1.61 ± 0.13 g/L of TUDCA from 3.23 g/L of TCDCA at a ratio of 1.3 to TCDCA. This study provides a potential approach for bear bile substitute production from cheap and readily available chicken bile.





Similar content being viewed by others
References
Li S, Tan HY, Wang N et al (2016) Substitutes for bear bile for the treatment of liver diseases: research progress and future perspective. Evidence-Based Complement Altern Med 2016:1–10. doi:10.1155/2016/4305074
Feng Y, Siu K, Wang N et al (2009) Bear bile: dilemma of traditional medicinal use and animal protection. J Ethnobiol Ethnomed 5:2. doi:10.1186/1746-4269-5-2
Hofmann AF, Hagey LR (2008) Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci 65:2461–2483. doi:10.1007/s00018-008-7568-6
Qiao X, Song W, Lin XH et al (2014) Rapid chemical analysis of bear bile: 5 minute separation and quantitation of bile acids using UHPLC-qTOF-MS. Anal Methods 6:596–601. doi:10.1039/c3ay41605d
Niu X, Xu Y, Yang Q et al (2014) Analytical methods for characterization of bile acids and its application in quality control of cow-bezoar and bear bile powder. Am J Appl Chem 2:96–104. doi:10.11648/j.ajac.20140206.11
Zhang D, Zhang N (1994) Comparing study on the composition of chicken bile and snake bile. Chin J Biochem Pharm 15:4–7 (in Chinese)
Yeh YH, Hwang DF (2001) High-performance liquid chromatographic determination for bile components in fish, chicken and duck. J Chromatogr B Biomed Sci Appl 751:1–8. doi:10.1016/S0378-4347(00)00448-5
Qiao X, Ye M, Pan DL et al (2011) Differentiation of various traditional Chinese medicines derived from animal bile and gallstone: simultaneous determination of bile acids by liquid chromatography coupled with triple quadrupole mass spectrometry. J Chromatogr A 1218:107–117. doi:10.1016/j.chroma.2010.10.116
Momose T, Tsubaki T, Iida T, Nambara T (1997) An improved synthesis of taurine- and glycine-conjugated bile acids. Lipids 32:775–778. doi:10.1007/s11745-997-0099-8
Fedorowski T, Salen G, Tint GS, Mosbach E (1979) Transformation of chenodeoxycholic acid and ursodeoxycholic acid by human intestinal bacteria. Gastroenterology 77:1068–1073. doi:10.1016/S0016-5085(79)80079-7
Hirano S, Masuda N (1981) Epimerization of the 7-hydroxy group of bile acids by the combination of two kinds of microorganisms with 7 alpha- and 7 beta-hydroxysteroid dehydrogenase activity, respectively. J Lipid Res 22:1060–1068
Carballeira JD, Quezada MA, Hoyos P et al (2009) Microbial cells as catalysts for stereoselective red-ox reactions. Biotechnol Adv 27:686–714. doi:10.1016/j.biotechadv.2009.05.001
Chemler JA, Lim CG, Daiss JL, Koffas MAG (2010) A versatile microbial system for biosynthesis of novel polyphenols with altered estrogen receptor binding activity. Chem Biol 17:392–401. doi:10.1016/j.chembiol.2010.03.010
Lim CG, Fowler ZL, Hueller T et al (2011) High-yield resveratrol production in engineered Escherichia coli. Appl Environ Microbiol 77:3451–3460. doi:10.1128/AEM.02186-10
Ferrandi EE, Bertolesi GM, Polentini F et al (2012) In search of sustainable chemical processes: cloning, recombinant expression, and functional characterization of the 7α- and 7β-hydroxysteroid dehydrogenases from Clostridium absonum. Appl Microbiol Biotechnol 95:1221–1233. doi:10.1007/s00253-011-3798-x
Lee J-Y, Arai H, Nakamura Y et al (2013) Contribution of the 7β-hydroxysteroid dehydrogenase from Ruminococcus gnavus N53 to ursodeoxycholic acid formation in the human colon. J Lipid Res 54:3062–3069. doi:10.1194/jlr.M039834
Braun M, Sun B, Anselment B, Weuster-Botz D (2012) Novel whole-cell biocatalysts with recombinant hydroxysteroid dehydrogenases for the asymmetric reduction of dehydrocholic acid. Appl Microbiol Biotechnol 95:1457–1468. doi:10.1007/s00253-012-4072-6
Sun B, Kantzow C, Bresch S et al (2013) Multi-enzymatic one-pot reduction of dehydrocholic acid to 12-keto-ursodeoxycholic acid with whole-cell biocatalysts. Biotechnol Bioeng 110:68–77. doi:10.1002/bit.24606
Riva S, Bovara R, Pasta P, Carrea G (1986) Preparative-scale regio- and stereospecific oxidoreduction of cholic acid and dehydrocholic acid catalyzed by hydroxysteroid dehydrogenases. J Org Chem 51:2902–2906. doi:10.1021/jo00365a009
Bovara R, Canzi E, Carrea G et al (1993) Enzymatic alpha/beta inversion of the C-7-hydroxyl of steroids. J Org Chem 58:499–501
Liu L, Braun M, Gebhardt G et al (2013) One-step synthesis of 12-ketoursodeoxycholic acid from dehydrocholic acid using a multienzymatic system. Appl Microbiol Biotechnol 97:633–639. doi:10.1007/s00253-012-4340-5
Zheng MM, Wang RF, Li CX, Xu JH (2015) Two-step enzymatic synthesis of ursodeoxycholic acid with a new 7β-hydroxysteroid dehydrogenase from Ruminococcus torques. Process Biochem 50:598–604. doi:10.1016/j.procbio.2014.12.026
Ji Q, Tan J, Zhu L et al (2016) Preparing tauroursodeoxycholic acid (TUDCA) using a double-enzyme-coupled system. Biochem Eng J 105:1–9. doi:10.1016/j.bej.2015.08.005
Xu P, Vansiri A, Bhan N, Koffas MAG (2012) ePathBrick: a synthetic biology platform for engineering metabolic pathways in E. coli. ACS Synth Biol 1:256–266. doi:10.1021/sb300016b
Yoshimoto T, Higashi H, Kanatani A et al (1991) Cloning and sequencing of the 7 alpha-hydroxysteroid dehydrogenase gene from Escherichia coli HB101 and characterization of the expressed enzyme. J Bacteriol 173:2173–2179
Zhao S, Jones JA, Lachance DM et al (2015) Improvement of catechin production in Escherichia coli through combinatorial metabolic engineering. Metab Eng 28:43–53. doi:10.1016/j.ymben.2014.12.002
Chemler JA, Lock LT, Koffas MAG, Tzanakakis ES (2007) Standardized biosynthesis of flavan-3-ols with effects on pancreatic beta-cell insulin secretion. Appl Microbiol Biotechnol 77:797–807. doi:10.1007/s00253-007-1227-y
Yang L, Xiong A, He Y et al (2008) Bile acids metabonomic study on the CCl4- and alpha-naphthylisothiocyanate-induced animal models: quantitative analysis of 22 bile acids by ultraperformance liquid chromatography–mass spectrometry. Chem Res Toxicol 21:2280–2288. doi:10.1021/tx800225q
Keasling JD (2011) Synthetic biology for synthetic fuels. Aspen Ideas Festiv 3:64–76. doi:10.1021/cb7002434
Jones JAA, Koffas MAG (2016) Optimizing metabolic pathways for the improved production of natural products. Methods Enzymol. 575:179–193. doi:10.1016/bs.mie.2016.02.010
Yan Y, Li Z, Koffas MAG (2008) High-yield anthocyanin biosynthesis in engineered Escherichia coli. Biotechnol Bioeng 100:126–140. doi:10.1002/bit.21721
Xu P, Gu Q, Wang W et al (2013) Modular optimization of multi-gene pathways for fatty acids production in E. coli. Nat Commun 4:1409. doi:10.1038/ncomms2425
Zhao SJ, Hu ZB, Liu D, Leung FCC (2006) Two divergent members of 4-coumarate: coenzyme a ligase from Salvia miltiorrhiza bunge: cDNA cloning and functional study. J Integr Plant Biol 48:1355–1364. doi:10.1111/j.1744-7909.2006.00302.x
Xu P, Rizzoni EA, Sul S-Y, Stephanopoulos G (2016) Improving metabolic pathway efficiency by statistical model-based multivariate regulatory metabolic engineering. ACS Synth Biol 6(1):148–158. doi:10.1021/acssynbio.6b00187
Acknowledgements
This study was funded by the National Science and Technology Major Projects (2014ZX09301306-007) and National Science Foundation of China (No. 81673540). We greatly appreciate Professor Zhibi Hu and Mrs Jiyan Zhou, Shanghai University of Traditional Chinese Medicine, for their valuable comments and suggestions, and the company of Shanghai Kaibao Pharmaceutica Co. Ltd., China for providing chicken bile and the standard reference of bile acids.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, J., Wang, J., Yu, L. et al. Rapidly directional biotransformation of tauroursodeoxycholic acid through engineered Escherichia coli . J Ind Microbiol Biotechnol 44, 1073–1082 (2017). https://doi.org/10.1007/s10295-017-1935-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1935-y