Abstract
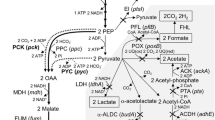
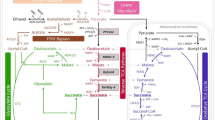
Actinobacillus succinogenes 130Z naturally produces among the highest levels of succinate from a variety of inexpensive carbon substrates. A few studies have demonstrated that A. succinogenes can anaerobically metabolize glycerol, a waste product of biodiesel manufacture and an inexpensive feedstock, to produce high yields of succinate. However, all these studies were performed in the presence of yeast extract, which largely removes the redox constraints associated with fermenting glycerol, a highly reduced molecule. We demonstrated that A. succinogenes cannot ferment glycerol in minimal medium, but that it can metabolize glycerol by aerobic or anaerobic respiration. These results were expected based on the A. succinogenes genome, which encodes respiratory enzymes, but no pathway for 1,3-propanediol production. We investigated A. succinogenes’s glycerol metabolism in minimal medium in a variety of respiratory conditions by comparing growth, metabolite production, and in vitro activity of terminal oxidoreductases. Nitrate inhibited succinate production by inhibiting fumarate reductase expression. In contrast, growth in the presence of dimethylsulfoxide and in microaerobic conditions allowed high succinate yields. The highest succinate yield was 0.75 mol/mol glycerol (75 % of the maximum theoretical yield) in continuous microaerobic cultures. A. succinogenes could also grow and produce succinate on partially refined glycerols obtained directly from biodiesel manufacture. Finally, by expressing a heterologous 1,3-propanediol synthesis pathway in A. succinogenes, we provide the first proof of concept that A. succinogenes can be engineered to grow fermentatively on glycerol.




Similar content being viewed by others
References
Alexeeva S, de Kort B, Sawers G, Hellingwerf KJ, de Mattos MJ (2000) Effects of limited aeration and of the ArcAB system on intermediary pyruvate catabolism in Escherichia coli. J Bacteriol 182:4934–4940. doi:10.1128/JB.182.17.4934-4940.2000
Almeida JRM, Favaro LCL, Quirino BF (2012) Biodiesel biorefinery: opportunities and challenges for microbial production of fuels and chemicals from glycerol waste. Biotechnol Biofuels 5 doi:10.1186/1754-6834-5-48
Atlas RM (2005) In: Handbook of media for environmental microbiology. Taylor & Francis, Boca Raton, p 1152
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (eds) (1993) Current protocols in molecular biology. Greene Publishing & Wiley, New York
Biebl H, Menzel K, Zeng A-P, Deckwer W-D (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52:289–297
Bilous PT, Weiner JH (1985) Dimethyl sulfoxide reductase activity by anaerobically grown Escherichia coli HB101. J Bacteriol 162:1151–1155
Blankschien MD, Clomburg J, Gonzalez R (2010) Metabolic engineering of Escherichia coli for the production of succinate from glycerol. Metab Eng 12:409–419. doi:10.1016/j.ymben.2010.06.002
Boenigk R, Bowien S, Gottschalk G (1993) Fermentation of glycerol to 1,3-propanediol in continuous cultures of Citrobacter freundii. Appl Microbiol Biotechnol 38:453–457
Booth IR (2005) Glycerol and methylglyoxal metabolism. EcoSal Plus. doi:10.1128/ecosalplus.3.4.3
Carvalho M, Mato M, Roca C, Reis MA (2013) Succinic acid production from glycerol by Actinobacillus succinogenes using dimethylsulfoxide as electron acceptor. New Biotechnol S1871–S6784. doi:10.1016/j.nbt.2013.06.006
Cole J (1996) Nitrate reduction to ammonia by enteric bacteria: redundancy, or a strategy for survival during oxygen starvation? FEMS Microbiol Lett 136:1–11
Coursolle D, Gralnick JA (2010) Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1. Mol Microbiol 77:995–1008. doi:10.1111/j.1365-2958.2010.07266.x
Dickie P, Weiner JH (1979) Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem 57:813–821
Durnin G, Clomburg J, Yeates Z, Alvarez PJ, Zygourakis K, Campbell P, Gonzalez R (2009) Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnol Bioeng 103:148–161. doi:10.1002/bit.22246
Goh EB, Bledsoe PJ, Chen LL, Gyaneshwar P, Stewart V, Igo MM (2005) Hierarchical control of anaerobic gene expression in Escherichia coli K-12: the nitrate-responsive NarX-NarL regulatory system represses synthesis of the fumarate-responsive DcuS-DcuR regulatory system. J Bacteriol 187:4890–4899. doi:10.1128/jb.187.14.4890-4899.2005
González-Pajuelo M, Meynial-Salles I, Mendes F, Soucaille P, Vasconcelos I (2006) Microbial conversion of glycerol to 1,3-propanediol: physiological comparison of a natural producer, Clostridium butyricum VPI 3266, and an engineered strain, Clostridium acetobutylicum DG1(pSPD5). Appl Environ Microbiol 72:96–101. doi:10.1128/AEM.72.1.96-101.2006
Gottschalk G (1986) Bacterial metabolism, 2nd edn. Springer, New York
Joshi RV, Schindler BD, McPherson N, Tiwari K, Vieille C (2014) A markerless gene knockout method for Actinobacillus succinogenes 130Z based on natural transformation. Appl Environ Microbiol 80:3053–3061. doi:10.1128/AEM.00492-14
Kaspar HF, Tiedje JM (1981) Dissimilatory reduction of nitrate and nitrite in the bovine rumen: nitrous oxide production and effect of acetylene. Appl Environ Microbiol 41:705–709
Kim P, Laivenieks M, McKinlay J, Vieille C, Zeikus JG (2004) Construction of a shuttle vector for the overexpression of recombinant proteins in Actinobacillus succinogenes. Plasmid 51:108–115
Lee PC, Lee SY, Chang HN (2010) Kinetic study on succinic acid and acetic acid formation during continuous cultures of Anaerobiospirillum succiniciproducens grown on glycerol. Bioprocess Biosys Engin 33:465–471. doi:10.1007/s00449-009-0355-4
Lee PC, Lee SY, Hong SH, Chang HN (2002) Isolation and characterization of a new succinic acid-producing bacterium, Mannheimia succiniciproducens MBEL55E, from bovine rumen. Appl Microbiol Biotechnol 58:663–668. doi:10.1007/s00253-002-0935-6
Lee PC, Lee WG, Lee SY, Chang HN (2001) Succinic acid production with reduced by-product formation in the fermentation of Anaerobiospirillum succiniciproducens using glycerol as a carbon source. Biotechnol Bioeng 72:41–48. doi:10.1002/1097-0290(20010105)72:1<41:AID-BIT6>3.0.CO;2-N
Macfadyen LP, Redfield RJ (1996) Life in mucus: sugar metabolism in Haemophilus influenzae. Res Microbiol 147:541–551
McKinlay JB, Laivenieks M, Schindler BD, McKinlay AA, Siddaramappa S, Challacombe JF, Lowry SR, Clum A, Lapidus AL, Burkhart KB, Harkins V, Vieille C (2010) A genomic perspective on the potential of Actinobacillus succinogenes for industrial succinate production. BMC Genom 11:680. doi:10.1186/1471-2164-11-680
McKinlay JB, Vieille C (2008) 13C-metabolic flux analysis of Actinobacillus succinogenes fermentative metabolism at different NaHCO3 and H2 concentrations. Metab Eng 10:55–68. doi:10.1016/j.ymben.2007.08.004
McKinlay JB, Vieille C, Zeikus JG (2007) Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740. doi:10.1007/s00253-007-1057-y
McKinlay JB, Zeikus JG, Vieille C (2005) Insights into the Actinobacillus succinogenes fermentative metabolism in a chemically defined growth medium. Appl Environ Microbiol 71:6651–6656
Murarka A, Dharmadi Y, Yazdani SS, Gonzalez R (2008) Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl Environ Microbiol 74:1124–1135. doi:10.1128/AEM.02192-07
Park DH, Zeikus JG (1999) Utilization of electrically reduced neutral red by Actinobacillus succinogenes: physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J Bacteriol 181:2403–2410
Poole RK, Cook GM (2000) Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv Microb Physiol 43:165–224
Potter LC, Millington P, Griffiths L, Thomas GH, Cole JA (1999) Competition between Escherichia coli strains expressing either a periplasmic or a membrane-bound nitrate reductase: does Nap confer a selective advantage during nitrate-limited growth? Biochem J 344:77–84. doi:10.1042/0264-6021:3440077
Scholten E, Dägele D (2008) Succinic acid production by a newly isolated bacterium. Biotechnol Lett 30:2143–2146. doi:10.1007/s10529-008-9806-2
Schroder H, Haefner S, Von Abendroth G, Hollmann R, Raddatz A, Ernst H, Gurski H (2014) Microbial succinic acid producers and purification of succinic acid. US patent US 8,673,598 B2
Scholten E, Dägele D, Haefner S, Schröder H (2009) Microbial succinic acid producer Mannheimia succiniciproducens DD1
Scott RI, Yarlett N, Hillman K, Williams TN, Williams AG, Lloyd D (1983) The presence of oxygen in rumen liquor and its effects on methanogenesis. J Appl Bacteriol 55:143–149. doi:10.1111/j.1365-2672.1983.tb02658.x
Shalel-Levanon S, San KY, Bennett GN (2005) Effect of ArcA and FNR on the expression of genes related to the oxygen regulation and glycolysis pathway in Escherichia coli under microaerobic growth conditions. Biotechnol Bioeng 92:147–159. doi:10.1002/bit.20583
Shalel-Levanon S, San KY, Bennett GN (2005) Effect of oxygen on the Escherichia coli ArcA and FNR regulation systems and metabolic responses. Biotechnol Bioeng 89:556–564
Simon J (2002) Enzymology and bioenergetics of respiratory nitrite ammonification. FEMS Microbiol Rev 26:285–309. doi:10.1111/j.1574-6976.2002.tb00616.x
Stephanopoulos GN, Aristidou AA, Nielsen J (1998) Metabolic engineering. Principles and methodologies. Academic Press, London
Stewart V (1988) Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev 52:190–232
Tseng CP, Albrecht J, Gunsalus RP (1996) Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol 178:1094–1098
Unden G, Bongaerts J (1997) Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta 1320:217–234. doi:10.1016/S0005-2728(97)00034-0
Unden G, Dunnwald P (2008) The aerobic and anaerobic respiratory chain of Escherichia coli and Salmonella enterica: enzymes and energetics. EcoSal Plus. doi:10.1128/ecosalplus.3.2.2
van der Werf MJ, Guettler MV, Jain MK, Zeikus JG (1997) Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch Microbiol 167:332–342
Vlysidis A, Binns M, Webb C, Theodoropoulos C (2011) Glycerol utilisation for the production of chemicals: conversion to succinic acid, a combined experimental and computational study. Biochem Eng J 58–59:1–11. doi:10.1016/j.bej.2011.07.004
Wagner AFV, Schultz S, Bomke J, Pils T, Lehmann WD, Knappe J (2001) YfiD of Escherichia coli and Y061 of bacteriophage T4 as autonomous glycyl radical cofactors reconstituting the catalytic center of oxygen-fragmented pyruvate formate-lyase. Biochem Biophys Res Comm 285:456–462. doi:10.1006/bbrc.2001.5186
Weiner JH, MacIsaac DP, Bishop RE, Bilous PT (1988) Purification and properties of Escherichia coli dimethyl sulfoxide reductase, an iron-sulfur molybdoenzyme with broad substrate specificity. J Bacteriol 170:1505–1510
Werpy T, Petersen G (2004) Top value added chemicals from biomass: volume I-results of screening for potential candidates from sugars and synthetic gas. US Department of Energy
Yazdani SS, Gonzalez R (2007) Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18:213–219. doi:10.1016/j.copbio.2007.05.002
Zeikus JG, Jain MK, Elankovan P (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol 51:545–552
Zhang XL, Shanmugam KT, Ingram LO (2010) Fermentation of glycerol to succinate by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 76:2397–2401. doi:10.1128/AEM.02902-09
Acknowledgments
This work was supported by a grant from the Michigan Economic Development Corporation, by Michigan State University startup funds, and by grant # 2010-04061 from the US Department of Agriculture National Institute for Food and Agriculture’s Sustainable Bioenergy Research Program to CV. BS was supported in part by a research fellowship from the Michigan State University Quantitative Biology Initiative. We thank Reena Jain, Jean Kim, Maeva Bottex, and Abagail Gray for their technical assistance. We thank Drs. C. A. Reddy, Yair Shachar-Hill, Gemma Reguera, James McKinlay, Thomas Schmidt, and Clegg Waldron for helpful discussions; Joseph Leykam, Kermit Johnson, and Dr. A. Daniel Jones for technical assistance, Dr. Thomas Schmidt for use of his microaerobic chamber, and Dr. Eric Hegg for his comments on the manuscript. We are indebted to John Oakley from Michigan Biodiesel for providing us with samples of glycerol of various grades. All authors have agreed to submit this manuscript to the “Journal of Industrial Microbiology and Biotechnology”.
Conflict of interest
We do not have any financial relationship with the organizations that sponsored the research. We do not have any conflict of interest.
Ethical standards
The experiments described in this manuscript comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schindler, B.D., Joshi, R.V. & Vieille, C. Respiratory glycerol metabolism of Actinobacillus succinogenes 130Z for succinate production. J Ind Microbiol Biotechnol 41, 1339–1352 (2014). https://doi.org/10.1007/s10295-014-1480-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1480-x