Abstract
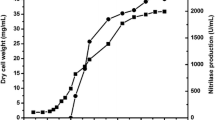
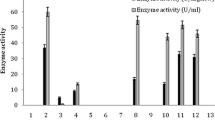
The acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674 has been applied to the conversion of benzamide and hydroxylamine to benzohydroxamic acid. The unique features of the acyl transfer activity of this organism include its optimal activity at 50 °C and very high substrate (100 mM benzamide) and product (90 mM benzohydroxamic acid) tolerance among the hitherto reported enzymes. The bench scale production of benzohydroxamic acid was carried out in a fed-batch reaction (final volume 1 l) by adding 50 mM benzamide and 250 mM of hydroxylamine after every 20 min for 80 min in 0.1 M potassium phosphate buffer (pH 7.0) at 50 °C, using resting cells equal to 4.0 mg dcm/ml of reaction mixture. From 1 l of reaction mixture 33 g of benzohydroxamic acid was recovered with 24.6 g l−1 h−1 productivity. The acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674 and the process developed in the present study are of industrial significance for the enzyme-mediated production of benzohydroxamic acid.








Similar content being viewed by others
References
Agrawal YK, Kunji PS (2005) Synthesis and dissociation constant of calix(6)arene hydroxamic acids. Iranian J Sci Technol 29:1–8
Bhalla TC, Kumar J, Kumar H, Agrawal HO (1997) Amidase production by Rhodococcus sp. NHB-2. Nat Acad Sci Lett 20:139–142
Brammar WJ, Clarke PH (1964) Induction and repression of Pseudomonas aeruginosa amidase. J Gen Microbiol 37:307–319
Dadd MR, Claridge TDW, Pettman AJ, Knowles CJ (2001) Biotransformation of benzonitrile to benzohydroxamic acid by Rhodococcus rhodochrous in the presence of hydroxylamine. Biotechnol Lett 23:221–225
Elford HL, Wampler GL, Van’t Riet B (1979) New ribonucleotide reductase inhibitors with antineoplastic activity. Cancer Res 39:844–851
Fournand D, Arnaud A (2001) Aliphatic and enantioselective amidases: from hydrolysis to acyl transfer activity. J Appl Microbiol 91:381–393
Fournand D, Bigey F, Arnaud A (1998) Acyl transfer activity of an amidase from Rhodococcus sp. Strain R312: formation of a wide range of hydroxamic acids. Appl Environ Microbiol 64:2844–2852
Holland KP, Howard L, Elford BV, Charles G, Annis SM, Schuster CD (1998) Antimalarial activities of polyhydroxyphenyl and hydroxamic acid derivatives. Antimicrob Agents Chemother 42:2456–2458
Holmes LB (1996) Hydroxamic acid: a potential human teratogen that could be recommended to treat ureaplasma. Teratology 53:227–229
Koncic MZ, Rajic Z, Petric N, Zorc B (2009) Antioxidant activity of NSAID hydroxamic acids. Acta Pharm 59:235–242
Kotlova EK, Chestukhina CG, Astaurova OB, Leonova TE, Yanenko AS, Debaboy VG (1999) Isolation and primary characterization of an amidase from Rhodococcus rhodochrous. Biochem Mosc 64:384–390
Miller MJ (1989) Syntheses and therapeutic potential of hydroxamic acid based siderophores and analogues. Chem Rev 89:1563–1579
Novo C, Farnaud S, Tata R, Clemente A, Brown PR (2002) Support for a three dimensional structure predicting a Cys-Glu-Lys catalytic triad for Pseudomonas aeruginosa amidase comes from site-directed mutagenesis and mutations altering substrate specificity. J Biochem 365:731–738
Rao MA, Scelza R, Scotti R, Gianfreda L (2010) Role of enzymes in the remediation of polluted environment. J Soil Sci Plant Nutr 10(3):333–353
Sharma NN, Sharma M, Bhalla TC (2009) Amidases: versatile enzymes in nature. Rev Environ Sci Biotechnol 8:343–366
Theiry A, Maestracci M, Arnaud A, Glazy P (1986) Acyl transfer activity of the wide spectrum amidase of Brevibacterium sp. R312. J Gen Microbiol 132:2205–2208
Valle NR, Gao S, Miller CP, Fulbright J, Gonzales C, Sirisawad M, Steggerda S, Wheler J, Balasubramanian S, Chandra J (2010) PCI-24781, a novel hydroxamic acid HDAC inhibitor, exerts cytotoxicity and histone alterations via caspase-8 and FADD in leukemia cells. Inter J Cell Biol 10:1155–1165
Vejvoda V, Martinkova L, Vesela AB, Kaplan O, Wahl SL, Fischerb L, Uhnakova B (2011) Biotransformation of nitriles to hydroxamic acids via a nitrile hydratase-amidase cascade reaction. J Mol Catal B Enzym 71:51–55
Acknowledgments
The authors are highly grateful to the New Delhi University Grant Commission and Department of Biotechnology (DBT) Ministry of Science and Technology New Delhi, India for providing financial assistance in the form of Junior Research Fellowship to Mr. Ravi Kant Bhatia and Senior Research Fellowship to Mr. Shashi Kant Bhatia and Mr. Praveen Kumar Mehta. The use of the computational facility at the Sub-Distributed Information Centre (SDIC), Himachal Pradesh University, Shimla is also duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatia, R.K., Bhatia, S.K., Mehta, P.K. et al. Bench scale production of benzohydroxamic acid using acyl transfer activity of amidase from Alcaligenes sp. MTCC 10674. J Ind Microbiol Biotechnol 40, 21–27 (2013). https://doi.org/10.1007/s10295-012-1206-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1206-x