Abstract
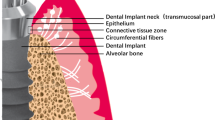
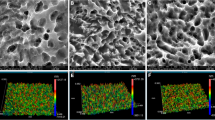
Connective tissue, one of the main components of peri-implant soft tissue, is key to the formation of the peri-implant mucosal seal and helping to prevent epithelial ingrowth. Rough surfaces (Rs), machined surfaces (Ms) or microgrooved surface (MG) are used in the neck area of commercially available titanium implants. In this paper, we aimed to evaluate the influence of surface topography of titanium substratum on connective tissue fibroblasts to gain a better understanding of this effect. Fibroblasts were cultured on titanium plates with Rs, Ms and MG. Adhesion cell number at day 3 was compared and protein distribution of both F-actin and vinculin was determined to observe cellular structure and adhesion. Cell adhesion strength was compared on each surface. At day 3, the number of fibroblasts attached on each substratum was in the order of MG ≈ Ms > Rs. Fibroblasts strongly expressed vinculin in the peripheral area on Ms and MG, and showed strong F-actin architecture. Decreased expression of vinculin and weaker continuity of F-actin were observed on Rs. Fibroblasts on MG were aligned along the grooves, with a significantly higher cell density, whereas cells on Ms and Rs had no clear orientation. The cell adhesion strength was significantly lower on Rs, and no significant difference was seen between MG and Ms. Both MG and Ms showed greater adhesion cell numbers and adhesion strength of fibroblasts when compared with Rs at day 3. The cell density on MG was greater than those on other substrata.










Similar content being viewed by others
References
Berglundh T, Lindhe J, Ericsson I, et al. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991;2:81–90.
Linkevicius T, Apse P. Biologic width around implants. An evidence-based review. Stomatologija. 2008;10:27–35.
Moon IS, Berglundh T, Abrahamsson I, Linder E, Lindhe J. The barrier between the keratinized mucosa and the dental implant. An experimental study in the dog. J Clin Periodontol. 1999;26:658–63.
Abrahamsson I, Zitzmann NU, Berglundh T, et al. The mucosal attachment to titanium implants with different surface characteristics: an experimental study in dogs. J Clin Periodontol. 2002;29:448–55.
Eisenbarth E, Meyle J, Nachtigall W, Breme J. Influence of the surface structure of titanium materials on the adhesion of fibroblasts. Biomaterials. 1996;17:1399–403.
Hamilton DW, Oakley C, Jaeger NA, Brunette DM. Directional change produced by perpendicularly oriented microgrooves is microtubule-dependent for fibroblasts and epithelium. Cell Motil Cytoskelet. 2009;66:260–71.
Loesberg WA, te Riet J, van Delft FC, et al. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials. 2007;28:3944–51.
Loesberg WA, Walboomers XF, Bronkhorst EM, van Loon JJ, Jansen JA. The effect of combined simulated microgravity and microgrooved surface topography on fibroblasts. Cell Motil Cytoskelet. 2007;64:174–85.
Seunarine K, Curtis AS, Meredith DO, et al. A hierarchical response of cells to perpendicular micro- and nanometric textural cues. IEEE Trans Nanobiosci. 2009;8:219–25.
Walboomers XF, Croes HJ, Ginsel LA, Jansen JA. Growth behavior of fibroblasts on microgrooved polystyrene. Biomaterials. 1998;19:1861–8.
Walboomers XF, Ginsel LA, Jansen JA. Early spreading events of fibroblasts on microgrooved substrates. J Biomed Mater Res. 2000;51:529–34.
Walboomers XF, Monaghan W, Curtis AS, Jansen JA. Attachment of fibroblasts on smooth and microgrooved polystyrene. J Biomed Mater Res. 1999;46:212–20.
Goodwin AE, Pauli BU. A new adhesion assay using buoyancy to remove non-adherent cells. J Immunol Methods. 1995;187:213–9.
Hormia M, Könönen M. Immunolocalization of fibronectin and vitronectin receptors in human gingival fibroblasts spreading on titanium surfaces. J Periodontal Res. 1994;29:146–52.
Campbell CE, von Recum AF. Microtopography and soft tissue response. J Invest Surg. 1989;2:51–74.
Dunn GA, Brown AF. Alignment of fibroblasts on grooved surfaces described by a simple geometric transformation. J Cell Sci. 1986;83:313–40.
Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20(Suppl 4):172–84.
Kokubu E, Hamilton DW, Inoue T, Brunette DM. Modulation of human gingival fibroblast adhesion, morphology, tyrosine phosphorylation, and ERK 1/2 localization on polished, grooved and SLA substratum topographies. J Biomed Mater Res A. 2009;91:663–70.
Kim SY, Oh N, Lee MH, et al. Surface microgrooves and acid etching on titanium substrata alter various cell behaviors of cultured human gingival fibroblasts. Clin Oral Implants Res. 2009;20:262–72.
Hormia M, Könönen M, Kivilahti J, Virtanen I. Immunolocalization of proteins specific for adhaerens junctions in human gingival epithelial cells grown on differently processed titanium surfaces. J Periodontal Res. 1991;26:491–7.
Könönen M, Hormia M, Kivilahti J, Hautaniemi J, Thesleff I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. J Biomed Mater Res. 1992;26:1325–41.
Mierke CT, Kollmannsberger P, Zitterbart DP, et al. Vinculin facilitates cell invasion into three-dimensional collagen matrices. J Biol Chem. 2010;285:13121–30.
Mierke CT, Kollmannsberger P, Zitterbart DP, et al. Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys J. 2008;94:661–70.
Goldmann WH, Schindl M, Cardozo TJ, Ezzell RM. Motility of vinculin-deficient F9 embryonic carcinoma cells analyzed by video, laser confocal, and reflection interference contrast microscopy. Exp Cell Res. 1995;221:311–9.
Subauste MC, Pertz O, Adamson ED, et al. Vinculin modulation of paxillin–FAK interactions regulates ERK to control survival and motility. J Cell Biol. 2004;165:371–81.
Saunders RM, Holt MR, Jennings L, et al. Role of vinculin in regulating focal adhesion turnover. Eur J Cell Biol. 2006;85:487–500.
Johnson RP, Craig SW. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261–4.
Acknowledgments
The authors express their sincere gratitude to Dr. Kanji Tsuru (Section of Biomaterials, Division of Oral Rehabilitation, Faculty of Dental Science, Kyushu University) for his technical support. The authors express their sincere gratitude to Dr. Yunia Dwi Rakhmatia (Division of Oral Rehabilitation, Faculty of Dental Science, Kyushu University), for her technical assistance. We appreciate for the technical support from the Research Support Center of the Graduate School of Medical Sciences, Kyushu University. This study was supported in part by KAKENHI (#21390520) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furuhashi, A., Ayukawa, Y., Atsuta, I. et al. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 100, 199–205 (2012). https://doi.org/10.1007/s10266-011-0029-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-011-0029-y