Abstract
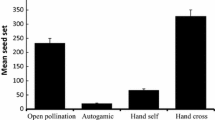
Ammopiptanthus mongolicus is an ecologically important species in the arid region of Northwest China. Habitat disturbance can significantly affect plant mating success and ultimately species viability. Pollen limitation of plant reproduction occurs in many plant species, particularly those under habitat disturbance. However, previous investigations have demonstrated differences in pollen limitation between conserved and disturbed sites. We compared the phenology, pollen limitation, pollinators and breeding system of both sites to determine whether habitat disturbance has generated changes in these plant components. We found that the species differed in four aspects. First, blooming duration and flowering peak were longer in the disturbed site than in the conserved site. Second, A. mongolicus can be pollen-limited and pollen limitation was more intense in the conserved site than in the disturbed site. Third, Anthophora uljanini was found to be a frequent pollinator in the conserved site, while Apis mellifera was the most effective and frequent flower visitor. More pollinator visits were recorded in the disturbed site, which could explain the differences in reproductive success. Finally, seed set was higher in the disturbed site than in the conserved site. We found that outcrossing was dominant in both sites and that agamospermy and self-pollination played complementary roles to ensure reproduction. Differences in flower production influenced by artificial selection and pollinator type explain the different seed set in both sites, whereas habitat disturbance cause changes differences in the pollination process and limits pollen flow. The balance between artificial management and mating success is crucial to analysis of the pollination process and manipulation of A. mongolicus population size.








Similar content being viewed by others
References
Aizen MA, Harder LD (2007) Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88:271–281
Aizen MA, Ashworth L, Galetto L (2002) Reproductive success in fragmented habitats: do compatibility systems and pollination specialization matter? J Veg Sci 13:885–892
Ashman TL, Morgan MT (2004) Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proc R Soc Lon B 271:553–559
Ashman TL et al (2004) Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421
Ashworth L, Aguilar R, Galetto L, Aizen MA (2004) Why do pollination generalist and specialist plant species show similar reproductive susceptibility to habitat fragmentation? J Ecol 92:717–719
Bond WJ (1994) Do mutualisms matter: assessing the impact of pollinator and disperser disruption on plant extinction. Philos Trans R Soc Lond B Biol Sci 344:83–90
Burd M (1994) Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot Rev 60:83–139
Byers DL (1995) Pollen quantity and quality as explanations for low seed set in small populations exemplified by Eupatorium (Asteraceae). Am J Bot 82:1000–1006
Camargo E, Rodrigues LC, Araujo AC (2011) Pollination biology and reproduction of Seemannia sylvatica (Kunth) Hanstein (Gesneriaceae) in the Serra da Bodoquena National Park, Mato Grosso do Sul. Biota Neotropica 4:125–130
Casper BB, Niesenbaum RA (1993) Pollen versus resource limitation of seed production: a reconsideration. Curr Sci India 65:210–214
Cosacov A, Nattero J, Cocucci AA (2008) Variation of pollinator assemblages and pollen limitation in a locally specialized system: the oil-producing Nierembergia linariifolia (Solanaceae). Ann Bot 102:723–734
Cruden RW (1977) Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 30:32–46
Cruden RW, Miller-Ward S (1981) Pollen–ovule ratio, pollen size and the ratio of stigmatic area to the pollen bearing area of the pollinator: a hypothesis. Evolution 35:964–974
Dafni A (1992) Pollination ecology: a practical approach. Oxford University Press, New York, pp 1–57
Dixon K (2009) Pollination and restoration. Science 325:571–573
Fang HT, Wang LY, Zhang XG (2004) A study of flower biology endangered plant Ammopiptanthus mongolicus. Guihaia 24:478–480
Fernández JD, Bosch J, Ariza N, Gómez JM (2012) Pollen limitation in a narrow endemic plant: geographical variation and driving factors. Oecologia 170:421–431
Galen C, Newport MEA (1988) Pollination quality, seed set, and flower traits in Polemonium viscosum—complementary effects of variation in flower scent and size. Am J Bot 75:900–905
García-Camacho R, Totland O (2009) Pollen limitation in the alpine: a meta-analysis. Arct Antarct Alp Res 41:103–111
Goddard M, Dougill A, Benton T (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25:90–98
Gómez JM, Abdelaziz M, Lorite J, Munõz-Pajares AJ, Perfectti F (2010) Changes in pollinator fauna cause spatial variation in pollen limitation. J Ecol 98:1243–1252
Goverde M, Schweizer K, Baur B, Erhardt A (2002) Small-scale habitat fragmentation effects on pollinator behaviour: experimental evidence from the bumblebee Bombus veteranus on calcareous grasslands. Biol Conserv 104:293–299
Haig D, Westoby M (1988) On limits to seed production. Am Nat 131:757–759
Herrera C (1987) Components of pollinator quality: comparative analysis of a diverse insect assemblage. Oikos 50:79–90
Hill LM, Brody AK, Tedesco CL (2008) Mating strategies and pollen limitation in a globally threatened perennial Polemonium vanbruntiae. Acta Oecol 33:314–323
Husband BC, Schemske DW (1996) Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50: 54–70
Janzen DH (1977) A note on optimal mate selection in plants. Am Nat 111:365–371
Jarne P, Charlesworth D (1993) The evolution of the selfing rate in functionally hermaphrodite plants and animals. Annu Rev Ecol Syst 24:441–466
Jennerston O (1988) Pollination in Dianthus deltoides (Caryophyllaceae): effects of habitat fragmentation on visitation and seed set. Conserv Biol 2:359–366
Kearns CA, Inouye DW (1993) Techniques for pollination biologists. University Press of Colorado Press, Colorado
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant–pollinator interactions. Annu Revecol Syst 29:83–112
Kevan PG, Clark EA, Thomas VG (1990) Insect pollinators and sustainable agriculture. Am J Altern Agr 5:13–22
Knight TM, Steet JA, Ashman TL (2006) A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am J Bot 93:271–277
Kudo G (1993) Relationships between flowering time and fruit set of the entomophilous alpine shrub, Rhododendron aureum (Ericaceae), inhabiting snow patches. Am J Bot 80:1300–1304
Larson BMH, Barrett SCH (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc 69:503–520
Li XW, Teng HK (1990) The flora of China. Science Press, Beijing, pp 140–142
Lloyd DG (1980) Demographic factors and mating patterns in angiosperms. In: Solbrig OT (ed) Demography and evolution in plant populations. University of California Press, Berkeley
Michalski SG, Durka W (2009) Pollination mode and life form strongly affect the relation between pollen/ovule ratios and the mating system. New Phytol 183:470–479
Oaxaca-Villa B, Casas A, Valiente-Banuet A (2006) Reproductive biology in wild and silvicultural managed populations of Escontria chiotilla (Cactaceae) in the Tehuacán Valley, Central México. Genet Resour Crop Evol 53:277–287
Oriani A, Scatena VL (2011) Reproductive biology of Abolboda pulchella and A. poarchon (Xyridaceae: Poales). Ann Bot 107:611–619
Ortíz FE, Stoner K, Pérez-Negrón E, Casas A (2010) Pollination biology of Myrtillocactus schenckii (Cactaceae) in wild and managed populations of the Tehuacán Valley, México. J Arid Environ 74:897–904
Pauw A, Bond WJ (2011) Mutualisms matter: pollination rate limits the distribution of oil-secreting orchids. Oikos 126:1531–1538
Rathcke BJ, Jules ES (1993) Habitat fragmentation and plant pollinator interactions. Bioscience 65:273–277
Revel N, Alvarez N, Gibernau M, Anahí E (2012) Investigating the relationship between pollination strategies and the size-advantage model in zoophilous plants using the reproductive biology of Arum cylindraceum and other European Arum species as case studies. Arthropod Plant Interact 6:35–44
Richards AJ (1997) Plant breeding systems. Chapman and Hall, London
Rodríguez-Oseguera AG, Casas A, Herrerías-Diego Y, Pérez-Negrón E (2013) Effect of habitat disturbance on pollination biology of the columnar cactus Stenocereus quevedonis at landscape-level in central Mexico. Plant Biol 15:573–582
Roubik DW (1996) Wild bees of Brunei-Darussalam. In: Edwards D, Choy S (eds) Tropcial rainforest research: current issues. Kluwer, The Netherlands
Roubik DW, Villanueva-Gutiérrez R (2009) Invasive Africanized honey bee impact on native solitary bees: a pollen resource and trap nest analysis. Biol J Linn Soc 98:152–160
Saunders D, Hobbs R, Margules C (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Spira TP, Snow AA, Whigham DF, Leak J (1992) Flower visitation, pollen deposition, and pollen-tube competition in Hibiscus moscheutos (Malvaceae). Am J Bot 79:428–433
Stuart W, Stephanie PL (2010) Reproduction of Echinacea angustifolia in fragmented prairie is pollen-limited but not pollinator-limited. Ecology 91:733–742
Wang YQ, Zhang DX, Chen ZY (2004) Pollen histochemistry and pollen: ovule ratios in Zingiberaceae. Ann B London 94:583–591
Wesselingh RA (2007) Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytol 174:26–37
Wiemer AP, Sérsic AN, Marino S, Cocucci AA (2012) Functional morphology and wasp pollination of two South American asclepiads (Asclepiadoideae–Apocynaceae). Ann Bot 109:77–93
Wilson P, Thomson JD, Stanton ML (1994) Beyond floral Batemania: gender biases in selection for pollination success. Am Nat 143:283–296
Young HJ, Stanton ML (1990) Temporal patterns of gamete production within individuals of Raphanus sativus (Brassicaceae). Can J Bot 68:480–486
Zapata TR, Arroyo MTK (1978) Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 10:221–230
Zych M, Stpiczyńska M (2012) Neither protogynous nor obligatory out-crossed: pollination biology and breeding system of the European Red List Fritillaria meleagris L. (Liliaceae). Plant Biol 14:285–294
Acknowledgments
This research was funded by Integrative research in key techniques for sustainable restoration of Sandy Land Ecosystems (2011BAC07B02), Research in techniques for risk assessment and prediction of Sandy Land Ecosystem (Y439K71001), “One Hundred Talent” Program of Chinese Academy of Sciences (Y451H31001) and National Natural Science Foundation of China (31300352, 41401620, 41501572). MC and XYZ conceived and designed the experiments. MC wrote the manuscript; other authors provided editorial advice. We thank Naiman Desertification Research Station and Urat Desert-grassland Research Station for all the help and support during this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, M., Zhao, XY., Zuo, XA. et al. Effects of habitat disturbance on the pollination system of Ammopiptanthus mongolicus (Maxim) Cheng f. at the landscape-level in an arid region of Northwest China. J Plant Res 129, 435–447 (2016). https://doi.org/10.1007/s10265-015-0779-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-015-0779-7