Abstract
Background
Deceased organ donations are rare in Japan, with most kidney transplants performed from a limited number of living donors. Researchers have thus developed highly successful ABO-incompatible transplantation procedures, emphasizing preoperative desensitization and postoperative immunosuppression. A recent open-label, single-arm, multicenter clinical study prospectively examined the efficacy and safety of rituximab/mycophenolate mofetil desensitization in ABO-incompatible kidney transplantation without splenectomy.
Methods
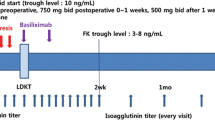
Mycophenolate mofetil and low dose steroid were started 28 days pretransplant, followed by two doses of rituximab 375 mg/m2 at day −14 and day −1, and postoperative immunosuppression with tacrolimus or ciclosporin and basiliximab. The primary endpoint was the non-occurrence rate of acute antibody-mediated rejection. Patient survival and graft survival were monitored for 1 year posttransplant.
Results
Eighteen patients received rituximab and underwent ABO-incompatible kidney transplantation. CD19-positive peripheral B cell count decreased rapidly after the first rituximab infusion and recovered gradually after week 36. The desensitization protocol was tolerable, and most rituximab-related infusion reactions were mild. No anti-A/B antibody-mediated rejection occurred with this series. One patient developed anti-HLA antibody-mediated rejection (Banff 07 type II) on day 2, which was successfully managed. Patient and graft survival were both 100 % after 1 year.
Conclusion
Our desensitization protocol was confirmed to be clinically effective and with acceptable toxicities for ABO-I-KTx (University Hospital Medical Information Network Registration Number: UMIN000006635).


Similar content being viewed by others
References
Takahashi K, Tanabe K, Ooba S, et al. Prophylactic use of a new immunosuppressive agent, deoxyspergualin, in patients with kidney transplantation from ABO-incompatible or preformed antibody-positive donors. Transplant Proc. 1991;23:1078.
Takahashi K, Saito K, Takahara S, et al. The Japanese ABO-incompatible kidney transplantation committee. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004;4:1089.
Takahashi K, Saito K. Present status of ABO-incompatible kidney transplantation in Japan. Xenotransplantation. 2006;13:118.
Ichimaru N, Takahara S. Japan’s experience with living-donor kidney transplantation across ABO barriers. Nat Clin Pract Nephrol. 2008;4:682.
Aikawa A, Saito K, Takahashi K. Trends in ABO-incompatible kidney transplantation. Exp Clin Transplant. 2015;13(Suppl 1):18.
Takahashi K, Saito K. ABO-incompatible kidney transplantation. Transplant Rev. 2013;27:1.
Takahashi K. ABO-incompatible kidney transplantation. Amsterdam: Elsevier; 2001.
Takahashi K. Accommodation in ABO-incompatible kidney transplantation. Amsterdam: Elsevier; 2004.
Takahashi K. ABO-incompatible kidney transplantation—establishing a scientific framework. Amsterdam: Elsevier; 2007.
Takahashi K. ABO-incompatible kidney transplantation—why is hyperacute rejection absent?. Amsterdam: Elsevier; 2011.
Takahashi K. ABO-incompatible kidney transplantation—overcoming hyperacute rejection and establishing clinical strategies. Amsterdam: Elsevier; 2013.
Tydén G, Kumlien G, Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76:730.
Alexandre GPJ, Bruyere MDE, Squifflet JP, Moriau M, Latinne D, Pirson Y. Human ABO-incompatible living donor renal homografts. Neth J Med. 1985;28:231.
Alexandre GPJ, Squifflet JP, Bruyère MDE, et al. ABO-incompatible related and unrelated living donor renal allografts. Transplant Proc. 1986;18:452.
Alexandre GPJ, Squifflet JP, Bruyère MDE, et al. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987;19:4538.
Alexandre GPJ, Latinne D, Gianello P, Squifflet JP. Preformed cytotoxic antibodies and ABO-incompatible grafts. Clin Transplant. 1991;5:583.
Takahashi K. Recent findings in ABO-incompatible kidney transplantation: classification and therapeutic strategy for acute antibody-mediated rejection due to ABO-blood-group-related antigens during the critical period preceding the establishment of accommodation. Clin Exp Nephrol. 2007;11:128.
Kobayashi T, Saito K. A series of surveys on assay for anti-A/B antibody by Japanese ABO-Incompatible Transplantation Committee. Xenotransplantation. 2006;13:136.
Takagi T, Ishida H, Shirakawa H, Shimizu T, Tanabe K. Evaluation of low-dose rituximab induction therapy in living related kidney transplantation. Transplantation. 2010;89:1466.
Fuchinoue S, Ishii Y, Sawada T, et al. The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction. Transplantation. 2011;91:853.
Shirakawa H, Ishida H, Shimizu T, et al. The low dose of rituximab in ABO-incompatible kidney transplantation without a splenectomy: a single-center experience. Clin Transplant. 2011;25:878.
Kohei N, Hirai T, Omoto K, Ishida H, Tanabe K. Chronic antibody-mediated rejection is reduced by targeting B-Cell immunity during an introductory period. Am J Transplant. 2012;12:469.
Sawada T, Fuchinoue S, Teraoka S. Successful A1-to-O ABO-incompatible kidney transplantation after a preconditioning regimen consisting of anti-CD20 monoclonal antibody infusions, splenectomy, and double-filtration plasmapheresis. Transplantation. 2002;74:1207.
Kabei K, Uchida J, Iwai T, et al. Late-onset neutropenia and acute rejection in ABO-incompatible kidney transplant recipients receiving rituximab and mycophenolate mofetil. Transpl Immunol. 2014;31:92.
Saito K, Nakagawa Y, Suwa M, et al. Pinpoint targeted immunosuppression: anti-CD20/MMF desensitization with anti-CD25 in successful ABO-incompatible kidney transplantation without splenectomy. Xenotransplantation. 2006;13:111.
Acknowledgments
The authors thank the patients and their families, the investigators and other physicians, and the nurses and laboratory technicians in the institutions participating in this multicenter trial. We are also grateful to Dr. Shinichi Nishi (Kobe University Graduate School of Medicine, Hyogo, Japan) and Dr. Hideki Ohdan (Hiroshima University, Hiroshima, Japan) for their critical review of the clinical data as members of the Independent Data and Safety Monitoring Committee, and to Yutaka Yamaguchi (Yamaguchi Pathology Laboratory, Chiba, Japan) and Asami Takeda (Nagoya Daini Red Cross Hospital, Aichi, Japan) for their histopathological review as members of the Central Pathology Review Committee.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding sources
This multicenter, prospective clinical study was sponsored by Zenyaku Kogyo, Tokyo, Japan. This study was initiated to satisfy the development requirements from the Japan Transplant Recipients Organization and a request from the Ministry of Health, Labour and Welfare under the system for resolution of unapproved drugs and off-label drug use. The study protocol was based on long-term survey results from the Japanese ABO Incompatible Transplantation Committee. The rationale for the desensitization protocol and immunosuppression was based on research supported by a Grant-in-Aid for Scientific Research No. (A)24249078 by the Japan Society for the Promotion of Science, the Ministry of Education, Culture, Sports, Science and Technology 2012–2014 (Corresponding Researcher: Kota Takahashi).
Conflict of interest
Honoraria: Kota Takahashi (Zenyaku Kogyo), Kazunari Tanabe (Chugai) and Kunio Morozumi (Novartis). Research Funding: Takashi Yagisawa (Astellas, Chugai and Novartis) and Kazunari Tanabe (Astellas). Subsidies or Donations: Kazunari Tanabe (Chugai). Travel fees, gifts, and others: Kota Takahashi (Zenyaku Kogyo) and Kazuhide Saito (Astellas, Chugai, Novartis and Zenyaku Kogyo).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB Approval Number at Niigata University: CH23-012) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Takahashi, K., Saito, K., Takahara, S. et al. Results of a multicenter prospective clinical study in Japan for evaluating efficacy and safety of desensitization protocol based on rituximab in ABO-incompatible kidney transplantation. Clin Exp Nephrol 21, 705–713 (2017). https://doi.org/10.1007/s10157-016-1321-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1321-5