Abstract
Background
ADPKD is a renal pathology caused by mutations of PKD1 and PKD2 genes, which encode for polycystin-1 (PC1) and polycystin-2 (PC2), respectively. PC1 plays an important role regulating several signal transducers, including cAMP and mTOR, which are involved in abnormal cell proliferation of ADPKD cells leading to the development and expansion of kidney cysts that are a typical hallmark of this disease. Therefore, the inhibition of both pathways could potentiate the reduction of cell proliferation enhancing benefits for ADPKD patients.
Methods
The inhibition of cAMP- and mTOR-related signalling was performed by Cl-IB-MECA, an agonist of A3 receptors, and rapamycin, respectively. Protein kinase activity was evaluated by immunoblot and cell growth was analyzed by direct cell counting.
Results
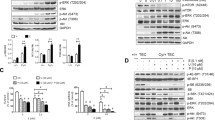
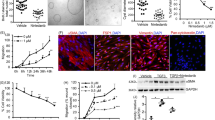
The activation of A3AR by the specific agonist Cl-IB-MECA causes a marked reduction of CREB, mTOR, and ERK phosphorylation in kidney tissues of Pkd1 flox/−: Ksp-Cre polycystic mice and reduces cell growth in ADPKD cell lines, but not affects the kidney weight. The combined sequential treatment with rapamycin and Cl-IB-MECA in ADPKD cells potentiates the reduction of cell proliferation compared with the individual compound by the inhibition of CREB, mTOR, and ERK kinase activity. Conversely, the simultaneous application of these drugs counteracts their effect on cell growth, because the inhibition of ERK kinase activity is lost.
Conclusion
The double treatment with rapamycin and Cl-IB-MECA may have synergistic effects on the inhibition of cell proliferation in ADPKD cells suggesting that combined therapies could improve renal function in ADPKD patients.




Similar content being viewed by others
References
Cornec-Le Gall E, et al. Genetics and pathogenesis of autosomal dominant polycystic kidney disease: 20 years on. Hum Mutat. 2014; 35:1393–1406.
Harris PC, Torres VE. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest. 2014;124:2315–24.
Serra AL, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–9.
Walz G, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–40.
Torres VE, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–18.
Caroli A, et al. ALADIN study group: effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382:1485–95.
Riella C, Czarnecki PG, Steinman TI. Therapeutic advances in the treatment of polycystic kidney disease. Nephron Clin Pract. 2014;128:297–302.
Muto S, et al. The effect of tolvaptan on autosomal dominant polycystic kidney disease patients: a subgroup analysis of the Japanese patient subset from TEMPO 3:4 trial. Clin Exp Nephrol. 2015;19:867–77.
Erickson KF, Chertow GM, Goldhaber-Fiebert JD. Cost-effectiveness of tolvaptan in autosomal dominant polycystic kidney disease. Ann Intern Med. 2013;17:382–9.
Brunelli SM, et al. End-stage renal disease in autosomal dominant polycystic kidney disease: a comparison of dialysis-related utilization and costs with other chronic kidney diseases. Clinicoecon Outcomes Res. 2015;7:65–72.
Aguiari G, Catizone L, Del Senno L. Multidrug therapy for polycystic kidney disease: a review and perspective. Am J Nephrol. 2013;37:175–82.
Loghman-Adham M, et al. Immortalized epithelial cells from human autosomal polycystic kidney cysts. Am. J. Physiol. Renal Physiol. 2003;285:F397–412.
Shibazaki S, et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet. 2008;17(11):1505–16.
Wodarczyk C, et al. Nephrocystin-1 forms a complex with polycystin-1 via a polyproline motif/SH3 domain interaction and regulates the apoptotic response in mammals. PLoS One. 2010;5:e12719.
Aguiari G, et al. Polycystin-1 regulates amphiregulin expression through CREB and AP1 signalling: implications in ADPKD cell proliferation. J Mol Med (Berl). 2012;90:1267–82.
Varani K, et al. Alteration of adenosine receptors in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:398–406.
Aguiari G, et al. Deficiency of polycystic kidney disease-1 gene (PKD1) expression increases A(3) adenosine receptors in human renal cells: implications for cAMP-dependent signalling and proliferation of PKD1-mutated cystic cells. Biochim Biophys Acta. 2009;1792:531–40.
Leuenroth SJ, et al. Triptolide reduces cystogenesis in a model of ADPKD. J Am Soc Nephrol. 2008;19:1659–62.
Shillingford JM, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–71.
Leonhard WN, et al. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol. 2011;300:F1193–202.
Zhong LM, et al. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS One. 2012;7(2):e32195.
Yamaguchi T, et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983–94.
Omori S, et al. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol. 2006;17(6):1604–14.
Hopp K, et al. Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J Am Soc Nephrol. 2015;26:39–47.
Mekahli D, et al. Polycystin-1 but not polycystin-2 deficiency causes upregulation of the mTOR pathway and can be synergistically targeted with rapamycin and metformin. Pflugers Arch. 2014;466:1591–604.
Acknowledgments
We greatly thanks Prof. PC Harris, (Mayo Clinic; Rochester, USA) for providing human normal and cystic kidney epithelial cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
de Stephanis, L., Bonon, A., Varani, K. et al. Double inhibition of cAMP and mTOR signalling may potentiate the reduction of cell growth in ADPKD cells. Clin Exp Nephrol 21, 203–211 (2017). https://doi.org/10.1007/s10157-016-1289-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1289-1