Abstract
Background
Several studies have demonstrated that spironolactone has an anti-albuminuric property in diabetic nephropathy. As an adverse event, spironolactone often induces the elevation of creatinine levels with hypotension and hyperkalemia. Therefore, we aimed to evaluate the efficacy and safety of spironolactone in Japanese patients with type 2 diabetes treated with either angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
Methods
Fifty-two Japanese patients with diabetic nephropathy and albuminuria (100 mg/gCr–2000 mg/gCr) treated with renin–angiotensin system (RAS) blockade were enrolled in a prospective, randomized, open-label study. The patients were subjected to add-on treatment with spironolactone 25 mg once daily and compared with matched controls for 8 weeks. The primary outcome was a reduction in the rate of albuminuria at 8 weeks compared with the baseline value. This study was registered with UMIN Clinical Trials Registry (000008016).
Results
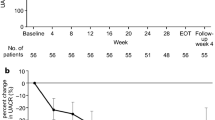
Albuminuria was reduced by 33 % (95 % confidence interval: 22–54; P = 0.0002) at 8 weeks with spironolactone. In the spironolactone group, blood pressure tended to lower and the estimated glomerular filtration rate (eGFR) was significantly decreased compared to those in the control group. When adjusted by systolic blood pressure and eGFR, spironolactone treatment still showed a significant effect on albuminuria reduction in a linear mixed model (coefficient ± standard error; 514.4 ± 137.6 mg/gCr, P < 0.0005). No patient was excluded from the study because of hyperkalemia.
Conclusions
Spironolactone reduced albuminuria along with conventional RAS inhibitors in patients with diabetic nephropathy. Our study suggests that spironolactone exerts anti-albuminuric effects independent of systemic hemodynamic alterations.

Similar content being viewed by others
References
International Diabetes Federation (2013) IDF Diabetes Altas, 6 ed. Brussels. pp 29–49.
Nathan DM, Bayless M, Cleary P, Genuth S, Gubitosi-Klug R, Lachin JM, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62(12):3976–86. doi:10.2337/db13-1093.
Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7. doi:10.1038/414782a.
Iseki K. Predictors of diabetic end-stage renal disease in Japan. Nephrol Carlton. 2005;10(Suppl):S2–6. doi:10.1111/j.1440-1797.2005.00447.x.
Nakai S, Iseki K, Itami N, Ogata S, Kazama JJ, Kimata N, et al. Overview of regular dialysis treatment in Japan (as of 31 December 2009). Ther Apher Dial. 2012;16(1):11–53. doi:10.1111/j.1744-9987.2011.01050.x.
Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, et al. Microalbuminuria reduction with telmisartan in normotensive and hypertensive Japanese patients with type 2 diabetes: a post hoc analysis of The Incipient to Overt: Angiotensin II Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) study. Hypertens Res. 2008;31(4):657–64. doi:10.1291/hypres.31.657.
Araki S, Haneda M, Koya D, Hidaka H, Sugimoto T, Isono M, et al. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes. 2007;56(6):1727–30. doi:10.2337/db06-1646.
Bilous R. Microvascular disease: what does the UKPDS tell us about diabetic nephropathy? Diabet Med. 2008;25(Suppl 2):25–9. doi:10.1111/j.1464-5491.2008.02496.x.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi:10.1056/NEJMoa0806470.
Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–8. doi:10.1056/NEJMoa011489.
Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care. 2007;30(6):1577–8. doi:10.2337/dc06-1998.
Chrysostomou A, Becker G. Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal disease. N Engl J Med. 2001;345(12):925–6. doi:10.1056/NEJM200109203451215.
Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20(12):2641–50. doi:10.1681/ASN.2009070737.
Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Rossing P, Tarnow L, et al. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int. 2005;68(6):2829–36. doi:10.1111/j.1523-1755.2005.00756.x.
Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005;28(9):2106–12.
Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70(12):2116–23. doi:10.1038/sj.ki.5001854.
Furumatsu Y, Nagasawa Y, Tomida K, Mikami S, Kaneko T, Okada N, et al. Effect of renin-angiotensin-aldosterone system triple blockade on non-diabetic renal disease: addition of an aldosterone blocker, spironolactone, to combination treatment with an angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker. Hypertens Res. 2008;31(1):59–67. doi:10.1291/hypres.31.59.
Konishi Y, Nishiyama A, Morikawa T, Kitabayashi C, Shibata M, Hamada M, et al. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension. 2011;58(2):205–11. doi:10.1161/HYPERTENSIONAHA.110.166843.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92. doi:10.1053/j.ajkd.2008.12.034.
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61(2):197–203. doi:10.1053/j.ajkd.2012.07.007.
Woo KS, Choi JL, Kim BR, Kim JE, An WS, Han JY. Urinary neutrophil gelatinase-associated lipocalin levels in comparison with glomerular filtration rate for evaluation of renal function in patients with diabetic chronic kidney disease. Diabetes Metab J. 2012;36(4):307–13. doi:10.4093/dmj.2012.36.4.307.
Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16(7):2073–80. doi:10.1681/ASN.2004080676.
Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008;51(2):199–211. doi:10.1053/j.ajkd.2007.10.040.
Morales E, Millet VG, Rojas-Rivera J, Huerta A, Gutierrez E, Gutierrez-Solis E, et al. Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol Dial Transplant. 2013;28(2):405–12. doi:10.1093/ndt/gfs429.
Esteghamati A, Noshad S, Jarrah S, Mousavizadeh M, Khoee SH, Nakhjavani M. Long-term effects of addition of mineralocorticoid receptor antagonist to angiotensin II receptor blocker in patients with diabetic nephropathy: a randomized clinical trial. Nephrol Dial Transplant. 2013;28(11):2823–33. doi:10.1093/ndt/gft281.
van den Meiracker AH, Baggen RG, Pauli S, Lindemans A, Vulto AG, Poldermans D, et al. Spironolactone in type 2 diabetic nephropathy: effects on proteinuria, blood pressure and renal function. J Hypertens. 2006;24(11):2285–92. doi:10.1097/01.hjh.0000249708.44016.5c.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17. doi:10.1056/NEJM199909023411001.
Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41(1):64–8.
Yoneda T, Takeda Y, Usukura M, Oda N, Takata H, Yamamoto Y, et al. Aldosterone breakthrough during angiotensin II receptor blockade in hypertensive patients with diabetes mellitus. Am J Hypertens. 2007;20(12):1329–33. doi:10.1016/j.amjhyper.2007.09.001.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nature medicine. 2008;14(12):1370–6. doi:10.1038/nm.1879.
Nishiyama A, Kobori H, Konishi Y, Morikawa T, Maeda I, Okumura M, et al. Mineralocorticoid receptor blockade enhances the antiproteinuric effect of an angiotensin II blocker through inhibiting podocyte injury in type 2 diabetic rats. The Journal of pharmacology and experimental therapeutics. 2010;332(3):1072–80. doi:10.1124/jpet.109.158113.
Chen H, Sun F, Zhong X, Shao Y, Yoshimura A, Liu Y. Eplerenone-mediated aldosterone blockade prevents renal fibrosis by reducing renal inflammation, interstitial cell proliferation and oxidative stress. Kidney Blood Press Res. 2013;37(6):557–66. doi:10.1159/000355736.
Ojeda-Cervantes M, Barrera-Chimal J, Alberu J, Perez-Villalva R, Morales-Buenrostro LE, Bobadilla NA. Mineralocorticoid receptor blockade reduced oxidative stress in renal transplant recipients: a double-blind, randomized pilot study. Am J Nephrol. 2013;37(5):481–90. doi:10.1159/000350539.
Ziyadeh FN, Goldfarb S. The renal tubulointerstitium in diabetes mellitus. Kidney Int. 1991;39(3):464–75.
Fujisawa G, Okada K, Muto S, Fujita N, Itabashi N, Kusano E, et al. Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int. 2004;66(4):1493–502. doi:10.1111/j.1523-1755.2004.00913.x.
Kramer AB, van der Meulen EF, Hamming I, van Goor H, Navis G. Effect of combining ACE inhibition with aldosterone blockade on proteinuria and renal damage in experimental nephrosis. Kidney Int. 2007;71(5):417–24. doi:10.1038/sj.ki.5002075.
Mavrakanas TA, Gariani K, Martin PY. Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: an emerging paradigm in diabetic nephropathy: a systematic review. Eur J Int Med. 2014;25(2):173–6. doi:10.1016/j.ejim.2013.11.007.
Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int. 2005;67(3):799–812. doi:10.1111/j.1523-1755.2005.00145.x.
Acknowledgments
This study included the following researchers. Principal investigator: Shoichi Maruyama. Steering Committee: Seiichi Matsuo, Hirofumi Makino, Enyu Imai, Takashi Uzu, Daisuke Koya and Yutaka Oiso. Data and Safety Monitoring Committee: Yukio Yuzawa and Mutsuharu Hayashi. Clinical Research Coordinator and Data Management Group: Masami Hamada, Kana Uchida, Miho Oba and Yumiko Omura.
Conflict of interest
This study was funded by Nagoya University Graduate School of Medicine. This study was supported in part by a Grant-in-Aid for Progressive Renal Diseases Research, Research on Rare and Intractable Disease, from the Ministry of Health, Labour and Welfare of Japan. The Department of Nephrology, Nagoya University Graduate School of Medicine, reported receiving research promotion grants from Astellas, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, Mochida, MSD, Nihon Medi-Physics, Novartis, Otsuka, Pfizer, Takeda, Teijin, Mitsubishi Tanabe and Torii. S.K. receives speaker honoraria from Novartis. Sh.M. receives speaker honoraria from Bayer, Chugai, Dainippon Sumitomo, Genzyme, Kowa, Kyowa Hakko Kirin, Mochida, MSD, Novartis, Otsuka, Public Health Research Center, Teijin and Mitsubishi Tanabe. Se.M. receives speaker honoraria from Alexon, Astellas, Baxter, Chugai, Daiichi Sankyo, Dainippon Sumitomo, Kaneka Medix, Kyowa Hakko Kirin, Mochida, MSD, Nihon Medi-Physics, Novartis, Otsuka, Public Health Research Center, Sanwa, Takeda, Teijin, Mitsubishi Tanabe and Torii. However, the research topics of these donation grants are not restricted. The Department of Medicine and Clinical Science, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences receives grant support from Astellas, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, MSD, Novo Nordisk, Pfizer, Takeda and Tanabe Mitsubishi. D.O. belongs to the Department of Diabetic Nephropathy, endowed by Boehringer Ingelheim, and receives grant support from Eli Lilly. J.W. is a consultant for Boehringer Ingelheim and receives speaker honoraria from Boehringer Ingelheim, Novartis and Novo Nordisk. H.M. is a consultant for AbbVie, Astellas and Teijin, and receives speaker honoraria from Astellas, MSD, Takeda and Tanabe Mitsubishi. However, the research topics of these donation grants are not restricted. Kanazawa Medical University receives donation from Pfizer and the donation is not directly associated with this study. Also, Kanazawa Medical University receives donation for research promotion from the following: MSD, Astellas, Kyowa Hakko Kirin, Daiichi Sankyo, Takeda, Mitsubishi Tanabe, Boehringer Ingelheim, Novartis and Japan Tobacco Inc. D.K. receives speaker honoraria from MSD, Astellas, Kyowa Hakko Kirin, Daiichi Sankyo, Takeda, Mitsubishi Tanabe, Boehringer Ingelheim, Novartis, Dainippon Sumitomo, Novo Nodisk, Sanofi, Kowa, Eli Lilly and Pfizer. K.K. receives speaker honoraria from, MSD, Astellas, Kyowa Hakko Kirin, Daiichi Sankyo, Mitsubishi Tanabe, Boehringer Ingelheim, Novartis, Dainippon Sumitomo, Sanofi and Eli Lilly. The Department of Medicine, Shiga University of Medical Science, reported receiving research promotion grants from Astellas, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Takeda, Teijin, Mitsubishi Tanabe and Chugai. T.U. receives speaker honoraria from MSD. The Department of Endocrinology and Diabetes, Nagoya University Graduate School of Medicine, reported receiving research promotion grants from Astellas, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, MSD, Kowa, Sanwa, Teijin, Mitsubishi Tanabe, Lilly and Novo Nordisk. Y.O. receives speaker honoraria from MSD and Ono. M.G. receives speaker honoraria from Astellas, Kowa, Mitsubishi Tanabe, Novo Nordisk, Takeda and Mochida. However, the research topics of these donation grants are not restricted. Department of Pharmacology, Faculty of Medicine, Kagawa University receives donation from Pfizer and the donation is not directly associated with this study. Center for Advanced Medicine and Clinical Research, Nagoya University Hospital, receives donation from Pfizer and the donation is not directly associated with this study. Pfizer organized the advisory meeting about aldosterone antagonist use in patients with diabetic nephropathy and S.K., Sh.M., H.M., T.U., K.K., E.I. and Se.M. were reimbursed for travel costs and received honoraria.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kato, S., Maruyama, S., Makino, H. et al. Anti-albuminuric effects of spironolactone in patients with type 2 diabetic nephropathy: a multicenter, randomized clinical trial. Clin Exp Nephrol 19, 1098–1106 (2015). https://doi.org/10.1007/s10157-015-1106-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1106-2