Abstract
Background
This multicenter study evaluated the feasibility of novel adjuvant chemotherapy with S-1 plus carboplatin followed by single-agent, long-term maintenance with S-1 in patients with completely resected stage II–IIIA non-small-cell lung cancer (NSCLC).
Methods
Patients received four cycles of S-1 (80 mg/m2/day for 2 weeks, followed by 2 weeks rest) plus carboplatin (area under the curve 5, day 1) followed by S-1 (80 mg/m2/day for 2 weeks, followed by a 1-week rest). Patients unable to continue S-1 plus carboplatin because of severe toxicity converted to single-agent S-1 maintenance. The duration of adjuvant chemotherapy was 10 months in both situations. The primary endpoint was feasibility, defined as the proportion of patients who completed four cycles of S-1 plus carboplatin and single-agent S-1 maintenance for 10 months. The treatment completion rate was determined; treatment was considered feasible if the lower 90% confidence interval (CI) was ≥50%.
Results
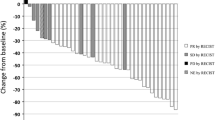
Eighty-nine patients were enrolled, of whom 87 were eligible and assessable. Seventy-eight patients (89.7%) completed four cycles of S-1 plus carboplatin and 55 (63.2%) completed the following S-1 maintenance therapy for a total of 10 months. The treatment completion rate was 63.2% (90% CI, 54.4–71.2%), indicating feasibility. There were no treatment-related deaths. Grade 3/4 toxicities included neutropenia (13.8%), thrombocytopenia (11.5%), and anorexia (4.6%). The 2-year relapse-free survival rate was 59.8%.
Conclusions
We concluded that adjuvant chemotherapy with S-1 plus carboplatin followed by single-agent maintenance therapy with S-1 is feasible and tolerable in patients with completely resected NSCLC.
Clinical registration number
UMIN000005041.



Similar content being viewed by others
References
The International Adjuvant Lung Cancer Trial Collaborative Group (2004) Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350:351–360
Douillard JY, Rosell R, De Lena M et al (2006) Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomized controlled trial. Lancet Oncol 7:719–727
Winton T, Livingston R, Johnson D et al (2005) Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352:2589–2597
Pignon JP, Tribodet H, Scagliotti GV et al (2008) Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 26:3552–3559
Kato H, Ichinose Y, Ohta M et al (2004) A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 350:1713–1721
Hamada C, Tsuboi M, Ohta M et al (2009) Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: an exploratory analysis from a meta-analysis of six randomized controlled trials. J Thorac Oncol 4:1511–1516
Wada H, Hitomi S, Teramatsu T (1996) Adjuvant chemotherapy after complete resection in non-small-cell lung cancer. West Japan study group for lung cancer surgery. J Clin Oncol 14:1048–1054
Hamada C, Tanaka F, Ohta M et al (2005) Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol 23:4999–5006
Shirasaka T, Nakano K, Takechi T et al (1996) Antitumoractivity of 1 M tegafur–0.4 M 5-chloro-2,4-dihydroxypyridine–1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56:2602–2606
Kawahara M, Furuse K, Segawa Y et al (2001) Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 85:939–943
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Uesaka K, Boku N, Fukutomi A et al (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. doi:10.1016/S0140-6736(16)30583-9 in press
Okamoto I, Yoshioka H, Morita S et al (2010) Phase III trial comparing oral S-1 plus carboplatin with paclitaxel plus carboplatin in chemotherapy-naïve patients with advanced non-small-cell lung cancer: results of a West Japan Oncology Group study. J Clin Oncol 28:5240–5246
Ciuleanu T, Brodowicz T, Zielinski C et al (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374:1432–1440
Cappuzzo F, Ciuleanu T, Stelmakh L et al (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11:521–529
Paz-Ares L, de Marinis F, Dediu M et al (2012) Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 13:247–255
Sobin LH, Gospodarowicz MK, Wittekind C (2009) TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, Hoboken
Basch E, Prestrud AA, Hesketh PJ et al (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29:4189–4198
Ettinger DS, Berger MJ, Armstrong DK et al (2015) Title of subordinate document. In: NCCN Version 2.2014, Antiemesis. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed Mar 2015
Kinoshita T, Nashimoto A, Yamamura Y et al (2004) Feasibility study of adjuvant chemotherapy with S-1 (TS-1; tegafur, gimeracil, oteracil potassium) for gastric cancer. Gastric Cancer 7:104–109
Tsuchiya T, Nagayasu T, Yamasaki N et al (2012) A multicenter phase II study of adjuvant chemotherapy with oral fluoropyrimidine S-1 for non-small-cell lung cancer: high completion and survival rates. Clin Lung Cancer 13:464–469
Tsukuda M, Kida A, Fujii M et al (2005) Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer 93:884–889
Yano T, Yamazaki K, Maruyama R et al (2010) Feasibility study of postoperative adjuvant chemotherapy with S-1 (tegafur, gimeracil, oteracil potassium) for non-small cell lung cancer–LOGIC 0601 study. Lung Cancer 67:184–187
Iwamoto Y, Mitsudomi T, Sakai K et al (2015) Randomized phase II study of adjuvant chemotherapy with long-term S-1 versus cisplatin + S-1 in completely resected stage II–IIIA non-small cell lung cancer. Clin Cancer Res 23:5245–5252
Niho S, Ikeda N, Michimae H et al (2013) Feasibility trial for adjuvant chemotherapy with docetaxel plus cisplatin followed by single agent long-term administration of S-1 chemotherapy in patients with completely resected non-small cell lung cancer: Thoracic Oncology Research Group study 0809. Br J Cancer 378:1–7
Acknowledgements
This work was supported, in part, by a non-profit organization, Epidemiological and Clinical Research Information Network (ECRIN). We are indebted to Ms. Yumi Miyashita (ECRIN) for data management, and Dr. Hideyuki Nishi (Okayama Rousai Hospital), Dr. Minoru Fukuda (Japanese Red Cross Nagasaki Genbaku Hospital), Dr. Shingo Harita (Chugoku Central Hospital of the Mutual Aid Association of Public School Teachers), Dr. Yoshifumi Sano (Ehime University Hospital), Dr. Masayoshi Teramachi (Osaka Saiseikai Noe Hospital), Dr. Shinji Kosaka (Shimane Prefectural Central Hospital), Dr. Takuji Fujinaga (Nagara Medical Center), Dr. Ryo Miyahara (Kyoto City Hospital), Dr. Eiji Miyahara (Saiseikai Hiroshima Hospital), Dr. Hidetoshi Inokawa (Mitoyo General Hospital), and Dr. Shinsuke Kajiwara (Uwajima City Hospital) for their contributions to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N.O., S.T., K.H., H.Y., and H.D. received honoraria from Taiho Pharmaceutical in Japan. All other authors declared no conflicts of interest regarding this study.
Additional information
Meeting presentation: 16th World Conference on Lung Cancer, Denver (Colorado, USA), 09/08/2015.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Okumura, N., Sonobe, M., Okabe, K. et al. Feasibility of adjuvant chemotherapy with S-1 plus carboplatin followed by single-agent maintenance therapy with S-1 for completely resected non-small-cell lung cancer: results of the Setouchi Lung Cancer Group Study 1001. Int J Clin Oncol 22, 274–282 (2017). https://doi.org/10.1007/s10147-016-1067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1067-9