Abstract
Background
Peripheral sensory neurotoxicity is a frequent adverse effect of oxaliplatin therapy. Calcium and magnesium (Ca/Mg) infusions are frequently used as preventatives, but a recent phase III trial failed to show that they prevent neurotoxicity. We therefore conducted a multicenter randomized phase III trial to compare fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) with and without Goshajinkigan (GJG), a traditional Japanese herbal medicine (Kampo), to determine GJG’s potential for reducing peripheral neuropathy in patients with colorectal cancer.
Methods
Patients with colon cancer who were undergoing adjuvant therapy with infusional mFOLFOX6 were randomly assigned to GJG (7.5 mg three times daily) or placebo in a double-blind manner. The primary endpoint was the time to grade 2 or greater neuropathy, which was determined at any point during or after oxaliplatin-based therapy using version 3 of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE).
Findings
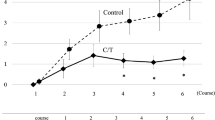
An interim analysis was performed when 142 of the planned 310 patients had been enrolled and the safety assessment committee recommended that the study be discontinued. One hundred eighty-two patients were evaluable for response. They included 89 patients in the GJG group and 93 patients in the placebo group. The incidence of grade 2 or greater neurotoxicity was 50.6 % in the GJG group and 31.2 % in the placebo group. A Cox proportional hazards analysis indicated that the use of GJG was significantly associated with the incidence of neuropathy (hazard ratio, 1.908; p = 0.007).
Conclusion
Goshajinkigan did not prevent oxaliplatin-associated peripheral neuropathy in this clinical trial. The clinical study was therefore terminated.



Similar content being viewed by others
References
Fuchs CS, Marshall J, Mitchell E et al (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25:4779–4786
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Tournigand C, Andre T, Achille E et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Van Cutsem E, Kohne CH, Hitre E et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Van Cutsem E, Peeters M, Siena S et al (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664
Andre T, Boni C, Mounedji-Boudiaf L et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
Tournigand C, Cervantes A, Figer A et al (2006) OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer––a GERCOR study. J Clin Oncol 24:394–400
Wen F, Zhou Y, Wang W et al (2013) Ca/Mg infusions for the prevention of oxaliplatin-related neurotoxicity in patients with colorectal cancer: a meta-analysis. Ann Oncol 24:171–178
Gamelin L, Boisdron-Celle M, Delva R et al (2004) Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res 10:4055–4061
Gamelin L, Boisdron-Celle M, Morel A et al (2008) Oxaliplatin-related neurotoxicity: interest of calcium-magnesium infusion and no impact on its efficacy. J Clin Oncol 26:1188–1189 (author reply 1189–1190)
Von Delius S, Eckel F, Wagenpfeil S et al (2007) Carbamazepine for prevention of oxaliplatin-related neurotoxicity in patients with advanced colorectal cancer: final results of a randomised, controlled, multicenter phase II study. Invest New Drugs 25:173–180
Milla P, Airoldi M, Weber G et al (2009) Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anticancer Drugs 20:396–402
Tawata M, Kurihara A, Nitta K et al (1994) The effects of goshajinkigan, a herbal medicine, on subjective symptoms and vibratory threshold in patients with diabetic neuropathy. Diabetes Res Clin Pract 26:121–128
Qin B, Nagasaki M, Ren M et al (2004) Gosha-jinki-gan (a herbal complex) corrects abnormal insulin signaling. Evid Based Complement Altern Med 1:269–276
Imamura T, Ishizuka O, Aizawa N et al (2008) Gosha-jinki-gan reduces transmitter proteins and sensory receptors associated with C fiber activation induced by acetic acid in rat urinary bladder. Neurourol Urodyn 27:832–837
Nishizawa M, Sutherland WH, Nukada H (1995) Gosha-jinki-gan (herbal medicine) in streptozocin-induced diabetic neuropathy. J Neurol Sci 132:177–181
Kaku H, Kumagai S, Onoue H et al (2012) Objective evaluation of the alleviating effects of goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: a multicenter collaborative study. Exp Ther Med 3:60–65
Ushio S, Egashira N, Sada H et al (2012) Goshajinkigan reduces oxaliplatin-induced peripheral neuropathy without affecting anti-tumour efficacy in rodents. Eur J Cancer 48:1407–1413
Kono T, Mamiya N, Chisato N et al (2011) Efficacy of goshajinkigan for peripheral neurotoxicity of oxaliplatin in patients with advanced or recurrent colorectal cancer. Evid Based Complement Altern Med 2011:418481
Japanese Society for Cancer of the Colon and Rectum (2009) Japanese Classification of Colorectal Carcinoma, 7th edn. Kanehara & Co., Ltd., Tokyo
Inoue N, Ishida H, Sano M et al (2012) Discrepancy between the NCI-CTCAE and DEB-NTC scales in the evaluation of oxaliplatin-related neurotoxicity in patients with metastatic colorectal cancer. Int J Clin Oncol 17:341–347
Sakurai M, Egashira N, Kawashiri T et al (2009) Oxaliplatin-induced neuropathy in the rat: involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain 147:165–174
Wu Z, Ouyang J, He Z et al (2012) Infusion of calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in colorectal cancer: a systematic review and meta-analysis. Eur J Cancer 48:1791–1798
Grothey A, Nikcevich DA, Sloan JA et al (2011) Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol 29:421–427
Loprinzi CL, Qin R, Dakhil SR et al (2014) Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol 32:997–1005
Uno T, Ohsawa I, Tokudome M et al (2005) Effects of goshajinkigan on insulin resistance in patients with type 2 diabetes. Diabetes Res Clin Pract 69:129–135
Usuki Y, Usuki S, Hommura S (1991) Successful treatment of a senile diabetic woman with cataract with goshajinkigan. Am J Chin Med 19:259–263
Mizuno K, Kono T, Suzuki Y et al (2014) Goshajinkigan, a traditional Japanese medicine, prevents oxaliplatin-induced acute peripheral neuropathy by suppressing functional alteration of TRP channels in rat. J Pharmacol Sci 125(1):91–98
Kono T, Mishima H, Shimada M et al (2009) Preventive effect of goshajinkigan on peripheral neurotoxicity of FOLFOX therapy: a placebo-controlled double-blind randomized phase II study (the GONE Study). Jpn J Clin Oncol 39:847–849
Hochster HS, Grothey A, Childs BH (2007) Use of calcium and magnesium salts to reduce oxaliplatin-related neurotoxicity. J Clin Oncol 25:4028–4029
Acknowledgments
We thank all patients who participated in this trial and their families. We are indebted to the physicians and all clinical study teams at the participating institutions. The following departments and hospitals participated in the trial: Department of Surgery and Science, Kyuhsu University (Fukuoka, Japan); Department of Surgery, Saiseikai Fukuoka General Hospital (Fukuoka, Japan); Department of Surgery, Minoh City Hospital, Minoh, Japan; Department of Gastroenterological Surgery, Aichi Cancer Center, Aichi Hospital (Nagoya, Japan);Division of Lower GI Department of Surgery, Hyogo College of Medicine (Nishinomiya, Japan); Department of Surgery, Kitasato University School of Medicine (Kanagawa, Japan); Kochi University Department of Surgery (Nankoku, Japan); Department of Surgery, Kurume University Medical Center (Kurume, Japan); Division of Gastroenterological Surgery, Chiba Cancer Center (Chiba, Japan); Department of Gastroenterological Surgery, Graduate School of Medical Sciences, Kumamoto University (Kumamoto, Japan); Department of Digestive Surgery, Breast and Thyroid Surgery, Graduate School of Medical Sciences, Kagoshima University (Kagoshima, Japan); Department of Surgery, Aomori Prefectural Central Hospital (Aomori City, Japan); Division of Surgical Oncology, Department of Translational Medical Sciences, Nagasaki University Graduate School of Biomedical Sciences (Nagasaki, Japan); Department of Surgery, University of Tokushima (Tokushima, Japan); Asahikawa Medical University Hospital (Asahikawa, Japan); Department of Surgery, Gifu Municipal Hospital (Gifu, Japan); Department of Gastroenterological Surgery, Nagoya University Graduate School of Medicine (Nagoya, Japan); Department of Surgery I, Dokkyo University School of Medicine (Shimotsuga, Japan); Department of Gastroenterological Surgery, Kyushu National Medical Center (Fukuoka, Japan); Japanese Red Cross Kanazawa Hospital (Kanazawa, Japan); First Department of Surgery, University of Fukui (Fukui, Japan); Department of Gastroenterological Surgery, Graduate School of Medicine, Osaka University (Osaka, Japan); First Department of Surgery, Ryukyu University, School of Medicine (Okinawa, Japan); Aichi Cancer Center Aichi Hospital; Department of Surgery, Fujita Health University (Toyoake, Japan); Department of General Surgery, Kawasaki Medical School (Okayama, Japan); Department of Surgical Oncology and Gastroenterological Surgery, Sapporo Medical University (Sapporo, Japan); Department of Gastroenterological Surgery, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Science (Okayama, Japan); Department of Surgical Oncology, Gifu University Graduate School of Medicine (Gifu, Japan); Hamamatsu University School of Medicine (Shizuoka, Japan); Department of Surgery, Kyoto University Graduate School of Medicine (Kyoto, Japan); Department of Gastroenterological Surgery, Hirosaki University Graduate School of Medicine (Hirosaki, Japan); Osaka Medical College (Osaka, Japan); Department of Surgery, Kansai Medical University (Osaka, Japan); Nihon University Itabashi Hospital (Tokyo, Japan); Kanazawa Medical University, Department of Surgical Oncology (Ishikawa, Japan); Department of Digestive Tract and General Surgery, Saitama Medical Center, Saitama Medical School (Saitama, Japan); Kansai Rosai Hospital (Amagasaki, Japan); Department of Gastrointestinal Surgery, Kobe University (Kobe, Japan). This clinical trial was totally supported by a Ministry of Health, Labour and Welfare Grant-in-Aid for Scientific Research in Japan (Tokyo, Japan). We also thank Ms. Satomi Abe and Ms. Masako Yamashita from the Kyushu University Data Center and the EPS Corporation (Tokyo, Japan) for their excellent secretarial assistance.
Conflict of interest
Yoshihiko Maehara received a research grant from Yakult Honsha and Tsumura & Co.; Toru Kono received a research Grant from Tsumura & Co; Eiji Oki and Takeshi Kato received lecture fees from Yakult Honsha; the other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10147_2015_784_MOESM1_ESM.pptx
Supplementary material 1. Time to grade 2 or greater sensory neuropathy (TTN), as measured according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE; version 3.0) and the Neurotoxicity Criteria of Debiopharm (DEB-NTC).
Fig. S1. A and B are the findings from the interim analysis. C and D are the findings from the final analysis. The black and dotted lines represent the Goshajinkigan (GJG) group and the placebo group, respectively
Fig. S2. A Time to grade 1 sensory neuropathy, reported by course. B Time to grade 2 sensory neuropathy, reported by course. C Time to grade 3 sensory neuropathy, reported by course. (PPTX 145 kb)
About this article
Cite this article
Oki, E., Emi, Y., Kojima, H. et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol 20, 767–775 (2015). https://doi.org/10.1007/s10147-015-0784-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0784-9