Abstract
Background
Imatinib mesylate, a small-molecule tyrosine kinase inhibitor, is currently used for adjuvant therapy of patients who have undergone resection of high-risk gastrointestinal stromal tumors (GISTs). There are no data concerning the efficacy and safety of postoperative adjuvant therapy with imatinib for Japanese or East Asian patients with GIST.
Methods
A single-arm, open-label, multicenter trial was conducted in 17 hospitals in Japan. The eligibility criteria included histologically proven primary high-risk GISTs with macroscopic complete resection. Patients were treated with imatinib at a dose of 400 mg/day for 1 year after surgery. The primary endpoint was recurrence-free survival as assessed by Kaplan–Meier analysis. The secondary endpoints were overall survival and safety. This study was registered with ClinicalTrials.gov, number NCT00171977.
Results
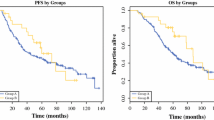
A total of 64 patients were enrolled between September 2004 and July 2006. The median age of the patients was 59.5 years. Forty-nine (76.6%) patients completed the 1-year treatment, whereas 15 (23.4%) patients did not complete the treatment owing to recurrence, toxicities, and consent withdrawal. At the median follow-up period of 109 weeks, 20 patients had recurrence. The 3-year recurrence rate was 42.7% (95% confidence interval 29.2–56.3%), which exceeded the expected recurrence rate in this trial. The recurrence-free and overall survival rates at 2 years were 71.1 and 93.7%, respectively. The most frequent adverse drug reaction of any grade was eyelid edema (48.4%), followed by neutropenia (40.6%), leukopenia (39.1%), nausea (39.1%), rash (37.5%), and peripheral edema (37.5%), most of which were mild and manageable.
Conclusions
Adjuvant therapy with imatinib at 400 mg/day for 1 year is well tolerated by Japanese patients and possibly reduces the risk of early recurrence of high-risk GISTs.




Similar content being viewed by others
References
Hirota S, Isozaki K, Moriyama Y et al (1998) Gain-of-function mutations of c-kit in human. Science 279:577–580
DeMatteo RP, Lewis JJ, Leung D et al (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58
Demetri GD, von Mehren M, Blanke CD et al (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472–480
Demetri GD, von Mehren M, Antonescu CR et al (2010) NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 8(Suppl 2):S1–S41
Kato H, Ichinose Y, Ohta M et al (2004) A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 350:1713–1721
Romond EH, Perez EA, Bryant J et al (2005) Trastumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
DeMatteo RP, Ballman KV, Antonescu CR et al (2009) Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 373:1097–1104
Nishida T, Shirao K, Sawaki A et al (2008) Efficacy and safety profile of imatinib mesylate (ST1571) in Japanese patients with advanced gastrointestinal stromal tumors: a phase II study (STI571B1202). Int J Clin Oncol 13:244–251
Fletcher CDM, Berman JJ, Corless C et al (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33:459–465
Jovanovic BD, Levy PS (1997) A look at the rule of three. Am Stat 51:137–139
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23:70–83
Heinrich MC, Corless CL, Demetri GD et al (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21:4342–4349
van Oosteron AT, Judson I, Verweij J et al (2001) Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumors: a phase I study. Lancet 358:1421–1423
Takahashi T, Nakajima K, Nishitani A et al (2007) An enhanced risk-group stratification system for more practical prognostication of clinically malignant gastrointestinal stromal tumors. Int J Clin Oncol 12:369–374
LeCesne A, Ray-Coquard I, Bui BN et al (2010) Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomized phase 3 trial. Lancet Oncol 11:942–949
Rutkowski P, Nowecki Z, Nyckowski P et al (2006) Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. J Surg Oncol 93:304–311
Casali PG, Blay JY (2010) Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(suppl 5):v98–v102
Kubota T (2006) Gastrointestinal stromal tumor (GIST) and imatinib. Int J Clin Oncol 11:184–189
Acknowledgments
This work was sponsored by Novartis Pharma K.K.
Conflict of interest
T. Nishida received lecture fees and research funding from Novartis Pharma, K.K.; T. Sugiyama and S. Hirota received research funding from Novartis Pharma, K.K.; all other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kanda, T., Nishida, T., Wada, N. et al. Adjuvant therapy with imatinib mesylate after resection of primary high-risk gastrointestinal stromal tumors in Japanese patients. Int J Clin Oncol 18, 38–45 (2013). https://doi.org/10.1007/s10147-011-0339-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0339-7