Abstract
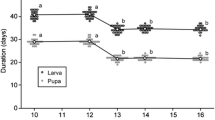
Reproductive interference is any interspecific sexual interaction that adversely affects female fitness through indiscriminate reproductive activities. It can be a driving force of resource partitioning in conjunction with resource competition. We previously showed that the bean beetle Callosobruchus maculatus is superior in larval resource competition, but vulnerable in reproductive interference, compared with its congener C. chinensis. We hypothesized that these two species might use two resources differently if one of the resources modified the intensity of reproductive interference or resource competition. We observed that C. maculatus females often enter gaps between beans to avoid mating attempts of heterospecific males, and hypothesized that removing bean gaps would strengthen reproductive interference. Therefore, we provided normal beans with gaps and split beans without gaps to females of the two species housed with conspecific or heterospecific males or no males and compared the number of eggs on each bean type among treatments. Callosobruchus maculatus females housed with heterospecific males were more likely to oviposit on normal beans than C. chinensis females. As a result, more C. chinensis adults hatched from split beans and more C. maculatus hatched from normal beans when females and males of both species were housed together. Thus, oviposition resource partitioning resulted from the combination of female avoidance of reproductive interference and resource competition.


Similar content being viewed by others
References
Avidov Z, Berlinger MJ, Applebaum SW (1965) Physiological aspects of host specificity in the Bruchidae: III. Effect of curvature and surface area on oviposition of Callosobruchus chinensis L. Anim Behav 13:178–180
Begon M, Harper JL, Townsend CR (2006) Ecology: from individuals to ecosystems, 4th edn. Blackwell Publishing, Malden
Bellows TS, Hassell MP (1984) Models for interspecific competition in laboratory populations of Callosobruchus spp. J Anim Ecol 53:831–848
Crowder DW, Horowitz AR, Breslauer H, Rippa M, Kontsedalov S, Ghanim M, Carrière Y (2011) Niche partitioning and stochastic processes shape community structure following whitefly invasions. Basic Appl Ecol 12:685–694
Forister ML (2004) Oviposition preference and larval performance within a diverging lineage of lycaenid butterflies. Ecol Entomol 29:264–272
Fox CW (1993) A quantitative genetic analysis of oviposition preference and larval performance on two hosts in the bruchid beetle, Callosobruchus maculatus. Evolution 47:166–175
Gröning J, Hochkirch A (2008) Reproductive interference between animal species. Q Rev Biol 83:257–282
Hochkirch A, Gröning J, Bücker A (2007) Sympatry with the devil: reproductive interference could hamper species coexistence. J Anim Ecol 76:633–642
Ishii S (1952) Studies of the host preference of the cowpea weevil (Callosobruchus chinensis L.). Bull Nat Inst Agr Sci Ser C 1:185–256
Kishi S, Nakazawa T (2013) Analysis of species coexistence co-mediated by resource competition and reproductive interference. Popul Ecol 55:305–313
Kishi S, Nishida T, Tsubaki Y (2009) Reproductive interference determines persistence and exclusion in species interactions. J Anim Ecol 78:1043–1049
Konuma J, Chiba S (2007) Ecological character displacement caused by reproductive interference. J Theor Biol 247:354–364
Kuno E (1992) Competitive exclusion through reproductive interference. Res Popul Ecol 34:275–284
Kyogoku D, Nishida T (2012) The presence of heterospecific males causes an Allee effect. Popul Ecol 54:391–395
Kyogoku D, Nishida T (2013) The mechanism of the fecundity reduction in Callosobruchus maculatus caused by Callosobruchus chinensis males. Popul Ecol 55:87–93
Liu S–S, De Barro PJ, Xu J, Luan J-B, Zang L-S, Ruan Y-M, Wan F-H (2007) Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 318:1769–1772
Miyatake T, Matsumura F (2004) Intra-specific variation in female remating in Callosobruchus chinensis and C. maculatus. J Insect Physiol 50:403–408
Noriyuki S, Osawa N, Nishida T (2011) Prey capture performance in hatchlings of two sibling Harmonia ladybird species in relation to maternal investment through sibling cannibalism. Ecol Entomol 36:282–289
Noriyuki S, Osawa N, Nishida T (2012) Asymmetric reproductive interference between specialist and generalist predatory ladybirds. J Anim Ecol 81:1077–1085
Ohsaki N, Sato Y (1994) Food plant choice of Pieris butterflies as a trade-off between parasitoid avoidance and quality of plants. Ecology 75:59–68
Okuzaki Y, Takami Y, Sota T (2010) Resource partitioning or reproductive isolation: the ecological role of body size differences among closely related species in sympatry. J Anim Ecol 79:383–392
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ribeiro JMC, Spielman A (1986) The satyr effect: a model predicting parapatry and species extinction. Am Nat 128:513–528
Runquist RB, Stanton ML (2013) Asymmetric and frequency-dependent pollinator-mediated interactions may influence competitive displacement in two vernal pool plants. Ecol Lett 16:183–190
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Shinoda K, Yoshida T (1990) Life history of the azuki bean weevil, Callosobruchus chinensis L. (Coleoptera: Bruchidae), in the field. In: Fujii K, Gatehouse AMR, Johnson CD, Mitchel R, Yoshida T (eds) Bruchids and legumes: economics, ecology and coevolution, Series Entomologica. Springer, Netherlands, pp 149–159
Soederbaeck B (1994) Reproductive interference between two co-occurring crayfish species, Astacus astacus L. and Pacifastacus leniusculus Dana. Nord J Freshwat Res 69:137–143
Takafuji A, Kuno E, Fujimoto H (1997) Reproductive interference and its consequences for the competitive interactions between two closely related Panonychus spider mites. Exp Appl Acarol 21:379–391
Takakura K-I, Fujii S (2010) Reproductive interference and salinity tolerance differentiate habitat use between two alien cockleburs: Xanthium occidentale and X. italicum (Compositae). Plant Ecol 206:309–319
Takakura K-I, Nishida T, Matsumoto T, Nishida S (2009) Alien dandelion reduces the seed-set of a native congener through frequency-dependent and one-sided effects. Biol Invasions 11:973–981
Thompson JN (1988) Coevolution and alternative hypotheses on insect/plant interactions. Ecology 69:893–895
Thum AR (2007) Reproductive interference, priority effects and the maintenance of parapatry in Skistodiaptomus copepods. Oikos 116:759–768
Toquenaga Y (1993) Contest and scramble competitions in Callosobruchus maculatus (Coleoptera: Bruchidae) II. Larval competition and interference mechanisms. Res Popul Ecol 35:57–68
Toquenaga Y, Fujii K (1991) Contest and scramble competitions in Callosobruchus maculatus (Coleoptera: Bruchidae). Res Popul Ecol 33:199–211
Umeya K (1987) Biology of bruchid beetles. Tsukiji Shokan, Tokyo
Utida S (1953) Interspecific competition between two species of bean weevil. Ecology 34:301–307
Wasserman SS (1986) Genetic variation in adaptation to foodplants among populations of the southern cowpea weevil, Callosobruchus maculatus: Evolution of oviposition preference. Entomol Exp Appl 42:201–212
Watanabe N (1985) Comparative studies on ecology of the azuki bean weevil, Callosobruchus chinensis (L.) and the cowpea weevil, C. maculatus (E.) (Coleoptera: Bruchidae). II. Relation between female body size and degree of creeping into the stored pile of seeds. Jpn J Appl Entomol Zool 29:107–112 (in Japanese with English summary)
Yoshida T (1966) Studies on the interspecific competition between bean weevils. Mem Fac Lib Arts Educ Univ Miyazaki 20:59–98
Yoshimura J, Clark CW (1994) Population dynamics of sexual and resource competition. Theor Popul Biol 45:121–131
Acknowledgments
We thank Noriyuki Suzuki, Takayoshi Nishida, and Tetsuro Yoshikawa for valuable comments on our previous drafts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kishi, S., Tsubaki, Y. Avoidance of reproductive interference causes resource partitioning in bean beetle females. Popul Ecol 56, 73–80 (2014). https://doi.org/10.1007/s10144-013-0390-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-013-0390-5