Abstract
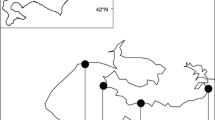
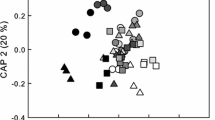
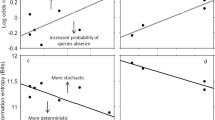
It is well known that the similarity in species composition between two communities decays with the geographic distance that separates them. It is thus likely that the similarity in the dynamics of two communities also decays with distance, because the distance–decay relationship is fundamental in nature. However, the distance–decay relationships of community dynamics have not yet been revealed. We used transition matrix models to evaluate distance–decay relationships of seasonal community dynamics (from spring to summer) in rocky intertidal sessile assemblages along the Pacific coast of Japan between 31°N and 43°N. We evaluated the distance–decay relationships of whole-community dynamics and of three dynamics-related components—recruitment, disturbance, and species interaction (competition and facilitation)—for communities separated by distances ranging from several meters to thousands of kilometers. The similarity of the recruitment dynamics among communities declined rapidly with distance within the fine spatial scale, but only moderately within larger scales. The similarity of the disturbance dynamics was independent of distance, and the similarity of species interaction declined slightly with increasing distance. The similarity of whole-community dynamics declined rapidly with distance at a fine spatial scale and moderately at larger scales. The fact that the distance–decay relationship of whole-community dynamics was similar to that of recruitment may suggest that recruitment processes are the most important determinant of spatial variability of community dynamics at our study sites during the study period.





Similar content being viewed by others
References
Aaviksoo K (1995) Simulating vegetation dynamics and land-use in a mire landscape using a Markov model. Landsc Urban Plan 31:129–142. doi:10.1016/0169-2046(94)01045-A
Asakura A (2003) Biogeography. In: Wada K (ed) Ecology of marine benthos. Tokai University Press, Kanagawa, pp 303–367
Condit R, Pitman N, Leigh EG, Chave J, Terborgh J, Foster RB, Nunez P, Aguilar S, Valencia R, Villa G, Muller-Landau HC, Losos E, Hubbell SP (2002) Beta-diversity in tropical forest trees. Science 295:666–669. doi:10.1126/science.1066854
Connell JH (1961) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42:710–723. doi:10.2307/1933500
Connell JH (1985) The consequences of variation in initial settlement vs postsettlement mortality in rocky intertidal communities. J Exp Mar Biol Ecol 93:11–15. doi:10.1016/0022-0981(85)90146-7
Connell JH, Keough MJ (1985) Disturbance and patch dynamics of subtidal marine animals on hard substrata. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic, Orlando, pp 125–151
Dayton PK (1971) Dispersion, dispersal, and persistence of the annual intertidal alga, Postelsia palmaeformis Ruprecht. Ecol Monogr 41:351–389. doi:10.2307/1948498
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman & Hall, London
Gaines S, Roughgarden J (1985) Larval settlement rate—a leading determinant of structure in an ecological community of the marine intertidal zone. Proc Natl Acad Sci USA 82:3707–3711. doi:10.1073/pnas.82.11.3707
Hawkins SJ, Hartnoll RG (1985) Factors determining the upper limits of intertidal canopy-forming algae. Mar Ecol Prog Ser 20:265–271. doi:10.3354/meps020265
Hill MF, Witman JD, Caswell H (2002) Spatio-temporal variation in Markov chain models of subtidal community succession. Ecol Lett 5:665–675. doi:10.1046/j.1461-0248.2002.00371.x
Hill MF, Witman JD, Caswell H (2004) Markov chain analysis of succession in a rocky subtidal community. Am Nat 164:E46–E61. doi:10.1086/422340
Hori M, Noda T (2001) An unpredictable indirect effect of algal consumption by gulls on crows. Ecology 82:3251–3256
Horn HS (1975) Markovian properties of forest succession. In: Cody ML, Diamond JM (eds) Ecology and evolution of communities. Harvard University Press, Cambridge, pp 196–211
Hutchinson N, Williams GA (2001) Spatio-temporal variation in recruitment on a seasonal, tropical rocky shore: the importance of local versus non-local processes. Mar Ecol Prog Ser 215:57–68. doi:10.3354/meps215057
Kinlan BP, Gaines SD (2003) Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84:2007–2020. doi:10.1890/01-0622
Koenig WD (1999) Spatial autocorrelation of ecological phenomena. Trends Ecol Evol 14:22–26. doi:10.1016/S0169-5347(98)01533-X
Lewin R (1986) Supply-side ecology. Science 234:25–27. doi:10.1126/science.234.4772.25
Littler MM, Littler DS (1980) The evolution of thallus form and survival strategies in benthic marine macroalgae field and laboratory tests of a functional form model. Am Nat 116:25–44. doi:10.1086/283610
Logofet DO, Lesnaya EV (2000) The mathematics of Markov models: what Markov chains can really predict in forest successions. Ecol Modell 126:285–298. doi:10.1016/S0304-3800(00)00269-6
Menge BA, Sutherland JP (1987) Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am Nat 130:730–757. doi:10.1086/284741
Minchinton TE, Scheibling RE (1993) Free-space availability and larval substratum selection as determinants of barnacle population-structure in a developing rocky intertidal community. Mar Ecol Prog Ser 95:233–244. doi:10.3354/meps095233
Morgan SG (2001) The larval ecology of marine communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland, pp 159–181
Morlon H, Chuyoun G, Condit R, Hubbell S, Kenfack D, Thomas D, Valencia R, Green JL (2008) A general framework for the distance-decay of similarity in ecological communities. Ecol Lett 11:904–917. doi:10.1111/j.1461-0248.2008.01202.x
Nakaoka M, Ito N, Yamamoto T, Okuda T, Noda T (2006) Similarity of rocky intertidal assemblages along the pacific coast of Japan: effects of spatial scales and geographic distance. Ecol Res 21:425–435. doi:10.1007/s11284-005-0138-6
Nekola JC, White PS (1999) The distance decay of similarity in biogeography and ecology. J Biogeogr 26:867–878. doi:10.1046/j.1365-2699.1999.00305.x
Noda T (2004a) Spatial hierarchical approach in community ecology: a way beyond high context dependency and low predictability in local phenomena. Popul Ecol 46:105–117. doi:10.1007/s10144-004-0184-x
Noda T (2004b) Large-scale variability in recruitment of the barnacle Semibalanus cariosus: its cause and effects on the population density and predator. Mar Ecol Prog Ser 278:241–252. doi:10.3354/meps278241
Noda T (2009) Metacommunity-level coexistence mechanisms in rocky intertidal sessile assemblages based on a new empirical synthesis. Popul Ecol 51:41–55. doi:10.1007/s10144-008-0117-1
Noda T, Minamiura N, Miyamoto Y (2003) Seasonal changes in an intertidal annual algal assemblage in northern Japan: the role of pre-emption and grazing on algal replacement. Ecol Res 18:695–709. doi:10.1111/j.1440-1703.2003.00589.x
Okuda T, Noda T, Yamamoto T, Ito N, Nakaoka M (2004) Latitudinal gradient of species diversity: multiscale variability in rocky intertidal sessile assemblages along the northwestern pacific coast. Popul Ecol 46:159–170. doi:10.1007/s10144-004-0190-z
Padilla DK (1985) Structural resistance of algae to herbivores—a biomechanical approach. Mar Biol (Berl) 90:103–109. doi:10.1007/BF00428220
Padilla DK, Allen BJ (2000) Paradigm lost: reconsidering functional form and group hypotheses in marine ecology. J Exp Mar Biol Ecol 250:207–221. doi:10.1016/S0022-0981(00)00197-0
Paine RT, Levin SA (1981) Intertidal landscapes: disturbance and the dynamics of pattern. Ecol Monogr 51:145–178. doi:10.2307/2937261
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. The Press Syndicate of the University of Cambridge, Cambridge
Raimondi PT (1988) Rock type affects settlement, recruitment, and zonation of the barnacle Chthamalus anisopoma Pilsbury. J Exp Mar Biol Ecol 123:253–267. doi:10.1016/0022-0981(88)90046-9
Reed DC, Laur DR, Ebeling AW (1988) Variation in algal dispersal and recruitment: the importance of episodic events. Ecol Monogr 58:321–335. doi:10.2307/1942543
Rindi F, Battelli C (2005) Spatio-temporal variability of intertidal algal assemblages of the Slovenian coast (Gulf of Trieste, northern Adriatic Sea). Bot Mar 48:96–105. doi:10.1515/BOT.2005.022
Roughgarden J, Gaines S, Possingham H (1988) Recruitment dynamics in complex life-cycles. Science 241:1460–1466. doi:10.1126/science.11538249
Shanks AL, Grantham BA, Carr MH (2003) Propagule dispersal distance and the size and spacing of marine reserves. Ecol Appl 13:S159–S169. doi:10.1890/1051-0761(2003)013[0159:PDDATS]2.0.CO;2
Shugart HH, Seagle SW (1985) Disturbance and patch dynamics on rocky intertidal shores. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic, Orlando, pp 353–368
Soininen J, McDonald R, Hillebrand H (2007) The distance decay of similarity in ecological communities. Ecography 30:3–12
Sousa WP (1979a) Disturbance in marine intertidal boulder fields: the nonequilibrium maintenance of species diversity. Ecology 60:1225–1239. doi:10.2307/1936969
Sousa WP (1979b) Experimental investigations of disturbance and ecological succession in a rocky inter-tidal algal community. Ecol Monogr 49:227–254. doi:10.2307/1942484
Sousa WP (1985) Disturbance and patch dynamics on rocky intertidal shores. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic, Orlando, pp 101–124
Sousa WP (2001) Natural disturbance and the dynamics of marine benthic communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland, pp 85–129
Spencer M (2006) Sensitivity analysis of Markov models for communities of competing sessile organisms. J Anim Ecol 75:1024–1033. doi:10.1111/j.1365-2656.2006.01124.x
Spencer M, Susko E (2005) Continuous-time Markov models for species interactions. Ecology 86:3272–3278. doi:10.1890/05-0029
Steneck RS (1982) A limpet-coralline alga association-adaptations and defenses between a selective herbivore and its prey. Ecology 63:507–522. doi:10.2307/1938967
Steneck RS, Dethier MN (1994) A functional-group approach to the structure of algal dominated communities. Oikos 69:476–498. doi:10.2307/3545860
Steneck RS, Watling L (1982) Feeding capabilities and limitation of herbivorous mollusks—a functional group approach. Mar Biol (Berl) 68:299–319. doi:10.1007/BF00409596
Tanner JE, Hughes TP, Connell JH (1994) Species coexistence, keystone species, and succession—a sensitivity analysis. Ecology 75:2204–2219. doi:10.2307/1940877
Tuomisto H, Ruokolainen K, Yli-Halla M (2003) Dispersal, environment, and floristic variation of western Amazonian forests. Science 299:241–244. doi:10.1126/science.1078037
Underwood AJ, Petraitis PS (1993) Structure of intertidal assemblages in different locations: how can local processes be compared? In: Ricklefs RE, Schluter D (eds) Species diversity in ecological communities: historical and geographical perspectives. The University of Chicago Press, Chicago, pp 39–51
Vadas RL, Johnson S, Norton TA (1992) Recruitment and mortality of early postsettlement stages of benthic algae. Br Phycol J 27:331–351. doi:10.1080/00071619200650291
Waggoner PE, Stephens GR (1970) Transition probabilities for a forest. Nature 225:1160–1161. doi:10.1038/2251160a0
Wootton JT (1993) Size-dependent competition—effects on the dynamics vs the end-point of mussel bed succession. Ecology 74:195–206. doi:10.2307/1939514
Wootton JT (2001a) Causes of species diversity differences: a comparative analysis of Markov models. Ecol Lett 4:46–56. doi:10.1046/j.1461-0248.2001.00190.x
Wootton JT (2001b) Predictions in complex communities: analysis of empirically derived Markov models. Ecology 82:580–598. doi:10.2307/2679881
Worm B, Lotze HK, Sommer U (2001) Algal propagule banks modify competition, consumer and resource control on Baltic rocky shores. Oecologia 128:281–293. doi:10.1007/s004420100648
Yoshizaki M (1979) Geographic distribution of marine algae of the Pacific coast of Japan, with special reference to algal flora of the Kii Peninsula. Mem Natl Sci Mus 12:201–211
Acknowledgments
We appreciate the generous support and encouragement of local fishermen and fishery officers of the Fisherman’s Cooperative Associations in Hokkaido, Iwate, Chiba, Wakayama, and Kagoshima Prefectures. We are grateful to the staff and students at Akkeshi Marine Station of Hokkaido University, International Coastal Research Center of Ocean Research Institute (The University of Tokyo), Marine Biosystems Research Center of Chiba University, Seto Marine Biological Laboratory of Kyoto University, and Education and Research Center for Marine Environment and Resources of Kagoshima University for field and laboratory facilities. We thank A. Aizawa and T. Hagino for useful discussion and comments, which helped to improve this paper, Dr. D. Munroe for critically reading and checking the English text, and two anonymous reviewers for comments and suggestions that improved a previous version of the manuscript. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (nos. 14340242 and 20570012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsujino, M., Hori, M., Okuda, T. et al. Distance decay of community dynamics in rocky intertidal sessile assemblages evaluated by transition matrix models. Popul Ecol 52, 171–180 (2010). https://doi.org/10.1007/s10144-009-0150-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-009-0150-8