Abstract
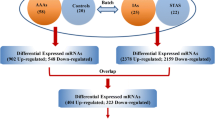
As it grows in size, an intracranial aneurysm (IA) is prone to rupture. In this study, we compared two extreme groups of IAs, ruptured IAs (RIAs) smaller than 10 mm and un-ruptured IAs (UIAs) larger than 10 mm, to investigate the genes involved in the facilitation and prevention of IA rupture. The aneurismal walls of 6 smaller saccular RIAs (size smaller than 10 mm), 6 larger saccular UIAs (size larger than 10 mm) and 12 paired control arteries were obtained during surgery. The transcription profiles of these samples were studied by microarray analysis. RT-qPCR was used to confirm the expression of the genes of interest. In addition, functional group analysis of the differentially expressed genes was performed. Between smaller RIAs and larger UIAs, 101 genes and 179 genes were significantly over-expressed, respectively. In addition, functional group analysis demonstrated that the up-regulated genes in smaller RIAs mainly participated in the cellular response to metal ions and inorganic substances, while most of the up-regulated genes in larger UIAs were involved in inflammation and extracellular matrix (ECM) organization. Moreover, compared with control arteries, inflammation was up-regulated and muscle-related biological processes were down-regulated in both smaller RIAs and larger UIAs. The genes involved in the cellular response to metal ions and inorganic substances may facilitate the rupture of IAs. In addition, the healing process, involving inflammation and ECM organization, may protect IAs from rupture.

Similar content being viewed by others
References
Chalouhi N, Ali MS, Jabbour PM et al (2012) Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab 32:1659–1676
Nieuwkamp DJ, Setz LE, Algra A et al (2009) Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 8:635–642
Greving JP, Wermer MJ, Brown RD Jr et al (2004) Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 13:59–66
Korja M, Lehto H, Juvela S (2014) Lifelong rupture risk of intracranial aneurysms depends on risk factors: a prospective Finnish cohort study. Stroke 45:1958–1963
Nakaoka H, Tajima A, Yoneyama T et al (2014) Gene expression profiling reveals distinct molecular signatures associated with the rupture of intracranial aneurysm. Stroke 45:2239–2245
Pera J, Korostynski M, Krzyszkowski T et al (2010) Gene expression profiles in human ruptured and un-rupturedintracranial aneurysms: what is the role of inflammation? Stroke 41:224–231
Marchese E, Vignati A, Albanese A et al (2010) Comparative evaluation of genome-wide gene expression profiles in ruptured and un-ruptured human intracranial aneurysms. Journal of Biological Regulators & Homeostatic Agents 24:185–195
Sawyer DM, Pace LA, Pascale CL et al (2016) Lymphocytes influence intracranial aneurysm formation and rupture: role of extracellular matrix remodeling and phenotypic modulation of vascular smooth muscle cells. J Neuroinflammation 13:1–9
Krischek B, Kasuya H, Tajima A et al (2008) Network-based gene expression analysis of intracranial aneurysm tissue reveals role of antigen presenting cells. Neuroscience 154:1398–1407
Platsoucas CD, Lu S, Nwaneshiudu I et al (2006) Abdominal aortic aneurysm is a specific antigen-driven T cell disease. Ann N Y Acad Sci 1085:224–235
Sweeney C, Morrow D, Birney YA et al (2004) Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a CBF-1/RBP-Jk dependent pathway. Faseb Journal Official Publication of the Federation of American Societies for Experimental Biology 18:1421–1423
Manon-Jensen T, Kjeld NG, Karsdal MA (2016) Collagen-mediated hemostasis. Journal of Thrombosis & Haemostasis 14:438–448
Li S, Wang D, Tian Y et al (2015) Aspirin inhibits degenerative changes of Aneurysmal Wall in a rat model. Neurochem Res 40:1537–1545
Conway DE, Lee S, Eskin SG et al (2010) Endothelial metallothionein expression and intracellular free zinc levels are regulated by shear stress. Am J Physiol Cell Physiol 299:C1461–C1467
Sarwar M, Samuel CS, Bathgate RA et al (2016) Enhanced serelaxin signalling in co-cultures of human primary endothelial and smooth muscle cells. Br J Pharmacol 173:484–496
Chalouhi N, Hoh BL, Hasan D (2013) Review of cerebral aneurysm formation, growth, and rupture. Stroke 44:3613–3622
Schulkens IA, Castricum KC, Weijers EM et al (2014) Expression, regulation and function of human metallothioneins in endothelial cells. J Vasc Res 51:231–238
Zbinden S, Wang J, Adenika R et al (2010) Metallothionein enhances angiogenesis and arteriogenesis by modulating smooth muscle cell and macrophage function. Arteriosclerosis Thrombosis & Vascular Biology 30:477–482
Can A, Du R (2016) Association of Hemodynamic Factors with Intracranial Aneurysm Formation and Rupture: systematic review and meta-analysis. Neurosurgery 78:510–520
Xiang J, Natarajan SK, Tremmel M et al (2011) Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke 42:144–152
Hosaka K, Hoh BL (2014) Inflammation and cerebral aneurysms. Translational Stroke Research 5:190–198
Hansson GK, Libby P, Schönbeck U et al (2002) Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res 91:281–291
Castro C, Campistol JM, Sancho D et al (2004) Rapamycin attenuates atherosclerosis induced by dietary cholesterol in apolipoprotein-deficient mice through a p27 Kip1 -independent pathway. Atherosclerosis 172:31–38
Yu H, Clarke MC, Figg N et al (2011) Smooth muscle cell apoptosis promotes vessel remodeling and repair via activation of cell migration, proliferation, and collagen synthesis. Arterioscler Thromb Vasc Biol 31:2402–2409
Hoh BL, Hosaka K, Downes DP et al (2011) Monocyte chemotactic protein-1 promotes inflammatory vascular repair of murine carotid aneurysms via a macrophage inflammatory protein-1α and macrophage inflammatory protein-2-dependent pathway. Circulation 124:2243–2252
Combs MD, Knutsen RH, Broekelmann TJ et al (2013) Microfibril-associated glycoprotein 2 (MAGP2) loss of function has pleiotropic effects in vivo. J Biol Chem 288:28869–28880
Starke RM, Chalouhi N, Ding D et al (2013) Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Translational Stroke Research 5:1–9
Peters DG, Kassam AB, Feingold E et al (2001) Molecular anatomy of an intracranial aneurysm: coordinated expression of genes involved in wound healing and tissue remodeling. Stroke 32:1036–1042
Shi C, Awad IA, Jafari N et al (2009) Genomics of human intracranial aneurysm wall. Stroke 40:1252–1261
Acknowledgments
We thank Dr. Zhe Xu for his assistance in analyzing the data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This research was supported by the cooperative project between The Science and Technology Ministry of China and Canada titled “Research on the genetics and pathogenesis of intracranial aneurysm and arteriovenous malformation” (No. 2010 dfb30850).
Conflicts of interest
The authors declare no potential conflicts of interest.
Ethical approval
This study was approved by the Ethics Committee of the Department of Medicine, Beijing Tiantan Hospital, Capital Medical University.
Informed consent
Informed consent was obtained from all patients.
Rights and permissions
About this article
Cite this article
Li, H., Li, H., Yue, H. et al. Comparison between smaller ruptured intracranial aneurysm and larger un-ruptured intracranial aneurysm: gene expression profile analysis. Neurosurg Rev 40, 419–425 (2017). https://doi.org/10.1007/s10143-016-0799-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-016-0799-3