Abstract
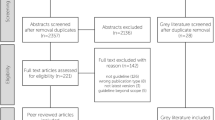
Clinical practice guidelines (CPGs) play an important role in healthcare. The guideline development process should be precise and rigorous to ensure that the results are reproducible and not vague. To determine the quality of guidelines, the Appraisal of Guidelines and Research and Evaluation (AGREE) instrument was developed and introduced. The aim of the present study was to assess the methodological quality of clinical practice guidelines on glioma. Eight databases (including MEDLINE and Embase) were searched till to August, 2013. The methodological quality of the guidelines was assessed by four authors independently using the AGREE II instrument. Fifteen relevant guidelines were included from 940 citations. The overall agreement among reviewers was moderate (intra-class correlation coefficient = 0.83; 95 % confidence interval [CI], 0.66–0.92). The mean scores were moderate for the domains “scope and purpose” (59.54) and “clarity of presentation” (65.46); however, there were low scores for the domains “stakeholder involvement” (43.80), “rigor of development” (39.01), “applicability” (31.89), and “editorial independence” (30.83). Only one third of the guidelines described the systematic methods for searching, and nearly half of the (47 %) guidelines did not give a specific recommendation. Only four of 15 described a procedure for updating the guideline; meanwhile, just six guidelines in this field can be considered to be evidence-based. The quality and transparency of the development process and the consistency in the reporting of glioma guidelines need to be improved. And the quality of reporting of guidelines was disappointing. Many other methodological disadvantages were identified. In the future, glioma CPGs should be based on the best available evidence and rigorously developed and reported. Greater efforts are needed to provide high-quality guidelines that serve as a useful and reliable tool for clinical decision-making in this field.

Similar content being viewed by others
References
AGREE Next Steps Consortium (2009) The AGREE II Instrument [Electronic version]. Available: http://www.agreetrust.org. Accessed 1 Jan 2011
Alberta Provincial CNS Tumour Team (2012) Glioblastoma. Edmonton (Alberta): Alberta Health Services, Cancer Care. (Clinical practice guideline; no. CNS-001 version 3)
Alonso-Coello P, Irfan A, Solà I, Gich I, Delgado-Noguera M, Rigau D, Tort S, Bonfill X, Burgers J, Schunemann H (2010) The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care 19(6):e58. doi:10.1136/qshc.2010.042077
Australian Cancer Network (2009) Adult Brain Tumour Guidelines Working Party. Clinical practice guidelines for the management of adult gliomas: astrocytomas and oligodendrogliomas. Cancer Council Australia, Australian Cancer Network and Clinical Oncological Society of Australia Inc., Sydney
Australian Institute of Health and Welfare and Australasian Association of Cancer Registries (2004) Cancer in Australia 2001. AIHW, Canberra
Berrocal A, Gil M, Gallego Ó, Balaña C, Pérez Segura P, García-Mata J, Reynes G, SEOM (Spanish Society of Clinical Oncology) (2012) SEOM guideline for the treatment of malignant glioma. Clin Transl Oncol 14(7):545–550
Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, Phillips B, Lelgemann M, Lethaby A, Bousquet J, Guyatt GH, Schünemann HJ, GRADE Working Group (2009) Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 64(5):669–677
Brozek JL, Akl EA, Jaeschke R, Lang DM, Bossuyt P, Glasziou P, Helfand M, Ueffing E, Alonso-Coello P, Meerpohl J, Phillips B, Horvath AR, Bousquet J, Guyatt GH, Schünemann HJ, GRADE Working Group (2009) Grading quality of evidence and strength of recommendations in clinical practice guidelines: Part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy 64(8):1109–1116
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L, AGREE next steps consortium (2010) AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 18:E839–E842
Burls A (2010) AGREE II—improving the quality of clinical care. Lancet 9747:1128–1129
Chamberlain MC, Kormanik PA (1998) Practical guidelines for the treatment of malignant gliomas. West J Med 168(2):114–120, 10
Cluzeau FA, Littlejohns P, Grimshaw JM, Feder G, Moran SE (1999) Development and application of a generic methodology to assess the quality of clinical guidelines. Int J Qual Health Care 11:21–28
Committee to advise the public health service on clinical practice guidelines, Institute of medicine (1990) In: Field MJ, Lohr KN (eds) Clinical practice guidelines: directions for a new program. National Academy Press, Washington (DC)
Davies E, Hopkins A (1997) Good practice in the management of adults with malignant cerebral glioma: clinical guidelines. Working Group, Royal College of Physicians. Br J Neurosurg 11(4):318–330
Dutch Society for Neuro-Oncology (2008) Gliomas. Amsterdam, The Netherlands: Association of Comprehensive Cancer Centres (ACCC). 28 p
Easaw JC, Mason WP, Perry J, Laperrière N, Eisenstat DD, Del Maestro R, Bélanger K, Fulton D, Macdonald D, Canadian Glioblastoma Recommendations Committee (2011) Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol 18(3):e126–e136
Fervers B, Burgers JS, Haugh MC, Latreille J, Mlika-Cabanne N, Paquet L, Coulombe M, Poirier M, Burnand B (2006) Adaptation of clinical guidelines: literature review and proposition for a framework and procedure. Int J Qual Health Care 18:167–176
Frappaz D, Chinot O, Bataillard A, Ben Hassel M, Capelle L, Chanalet S, Chatel M, Figarella-Branger D, Guegan Y, Guyotat J, Hoang-Xuan K, Jouanneau E, Keime-Guibert F, Laforêt C, Linassier C, Loiseau H, Maire JP, Menei P, Rousmans S, Sanson M, Sunyach MP, FNCLCC, Neuro-oncology Group of the Fédération Nationale des Centres de Lutte Contre le Cancer, Association of French-speaking Neuro-oncologists (2003) Summary version of the standards, options and recommendations for the management of adult patients with intracranial glioma. Br J Cancer 89(Suppl1):S73–S83
GRADE working group (2011) Available: http://www.gradeworkinggroup.org/news.htm. Accessed 23 Dec 2011
Grilli R, Magrini N, Penna A, Mura G, Liberati A (2000) Practice guidelines developed by specialty societies: the need for a critical appraisal. Lancet 355:103–106
Grol R, Cluzeau FA, Burgers JS (2003) Clinical practice guidelines: towards better quality guidelines and increased international collaboration. Br J Cancer 89((suppl 1)):S4–S8
Guidelines Glioma Working Group (2012) China clinical practice guidelines for diagnosis and treatment in central nervous system glioma. Natl Med J China 92(33):2309–2313
Hirsh J, Guyatt G (2009) Clinical experts or methodologists to write clinical guidelines? Lancet 374(9686):273–275. doi:10.1016/S0140-6736(09)60787-X. Epub 2009 Apr 23
Huang TW, Lai JH, Wu MY, Chen SL, Wu CH, Tam KW (2013) Systematic review of clinical practice guidelines in the diagnosis and management of thyroid nodules and cancer. BMC Med 11(1):191
Laperriere N, Walker-Dilks C (2009) PET imaging in brain cancer. Cancer Care Ontario, Toronto, Program in Evidence-based Care PET Recommendation Report No.:10
Mason WP, Maestro RD, Eisenstat D, Forsyth P, Fulton D, Laperrière N, Macdonald D, Perry J, Thiessen B, Canadian GBM Recommendations Committee (2007) Canadian recommendations for the treatment of glioblastoma multiforme. Curr Oncol 14(3):110–117
National Comprehensive Cancer Network (2011) NCCN clinical practice guidelines in oncology. Central nervous system cancers. Version 2. NCCN.org
Olson JJ, Fadul CE, Brat DJ, Mukundan S, Ryken TC (2009) Management of newly diagnosed glioblastoma: guidelines development, value and application. J Neurooncol 93(1):1–23. doi:10.1007/s11060-009-9838-z
Oxman AD, Glasziou P, Williams JW (2008) What should clinicians do when faced with conflicting recommendations? BMJ 337:a2530
Schünemann HJ, Hill SR, Kakad M, Vist GE, Bellamy R, Stockman L, Wisløff TF, Del Mar C, Hayden F, Uyeki TM, Farrar J, Yazdanpanah Y, Zucker H, Beigel J, Chotpitayasunondh T, Hien TT, Ozbay B, Sugaya N, Oxman AD (2007) Transparent development of the WHO rapid advice guidelines. PLoS Med 4:e119
Schünemann HJ, Osborne M, Moss J, Manthous C, Wagner G, Sicilian L, Ohar J, McDermott S, Lucas L, Jaeschke R, ATS Ethics and Conflict of Interest Committee and the Documents Development and Implementation Committee (2009) An official American Thoracic Society Policy statement: managing conflict of interest in professional societies. Am J Respir Crit Care Med 180(6):564–580. doi:10.1164/rccm.200901-0126ST
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 2:420–428
Shekelle P, Eccles MP, Grimshaw JM, Woolf SH (2001) When should clinical guidelines be updated? BMJ 323:155–157
Shekelle PG, Ortiz E, Rhodes S, Morton SC, Eccles MP, Grimshaw JM, Woolf SH (2001) Validity of the agency for healthcare research and quality clinical practice guidelines: how quickly do guidelines become outdated? JAMA 286:1461–1467
Soffietti R, Baumert BG, Bello L, von Deimling A, Duffau H, Frénay M, Grisold W, Grant R, Graus F, Hoang-Xuan K, Klein M, Melin B, Rees J, Siegal T, Smits A, Stupp R, Wick W, European Federation of Neurological Societies (2010) Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol 17(9):1124–1133. doi:10.1111/j.1468-1331.2010.03151.x
Stupp R, Tonn JC, Brada M, Pentheroudakis G, ESMO Guidelines Working Group (2010) High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v190-3. doi:10.1093/annonc/mdq187
Acknowledgments
The authors would like to acknowledge and extend their heartfelt gratitude to Tang Zhen from University of Bristol for her generous assistance and invaluable advice.
Conflict of interest
There is no financial support or relationships that may pose conflict of interest in this study paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Rao Sun, Lanzhou, China
Clinical practice guidelines are now a common feature of clinical practice and are of interest worldwide. They are expected to facilitate more consistent, effective, and efficient medical practice and improve health outcomes. Tian Hongliang et al. presented a thorough review which assessed the methodological quality of clinical practice guidelines on glioma. Fifteen relevant guidelines were included in eight databases till August, 2013. The review of published clinical practice guidelines revealed that the quality of reporting of guidelines was disappointing. The mean scores were low for the domains stakeholder involvement (43.80), rigor of development (39.01), applicability (31.89), and editorial independence (30.83). It points out that the future developers of glioma guideline need to pay more attention to these domains during the development process. They are expected to facilitate more consistent, effective, and efficient medical practice and improve health outcomes.
In all, the authors should be encouraged for their significant effort to provide a comprehensive and transparent picture of the glioma guideline. It is the first systematic review of guideline quality over the last 20 years. In the future, glioma guideline should base on the best available evidence and rigorously developed and reported. Greater efforts are needed to provide high-quality guidelines that serve as a useful and reliable tool for clinical decision-making in this field.
Tian Hongliang and Gou Yani contributed equally to this study.
Rights and permissions
About this article
Cite this article
Tian, H., Gou, Y., Pan, Y. et al. Quality appraisal of clinical practice guidelines on glioma. Neurosurg Rev 38, 39–47 (2015). https://doi.org/10.1007/s10143-014-0569-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-014-0569-z