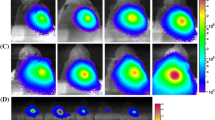
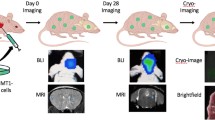
Abstract
Despite decades of study, the etiology of brain cancer remains elusive. However, extensive molecular characterization of primary brain tumors has been accomplished, outlining recurrent features that are proving useful for devising targeted therapies. There are far too few patients available for comparing the efficacy of therapeutic combinations, especially when variations in dosing, frequency, and sequencing are taken into account. Consequently, there is a substantial need for increasing preclinical testing throughput using clinically relevant models. We review luminescent optical imaging for its potential in facilitating in vivo assessment of intracranial tumor growth and response to therapy in rodent orthotopic xenograft models of primary brain malignancies. We review the rationale behind the need of an in vivo model, why orthotopic tumor models displaying an invasive phenotype may be a superior choice when compared to flank-implanted tumors, and what advantages may be drawn from the use of modified cells, suitable for sequential monitoring by in vivo optical imaging. Studies show that luminescent signal correlates highly both with tumor burden and Kaplan–Meier survival curves of rodents bearing intracranial xenografts. We conclude that bioluminescent imaging is a highly sensitive technique for assessment of tumor burden, response to therapy, tumor recurrence, and behavior to salvage therapy, making it a superior option for longitudinal monitoring in intracranial rodent models of primary brain tumors.


Similar content being viewed by others
References
Aldape KD, Ballman K, Furth A, Buckner JC, Giannini C, Burger PC et al (2004) Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol 63:700–707
Arcella A, Carpinelli G, Battaglia G, D'Onofrio M, Santoro F, Ngomba RT et al (2005) Pharmacological blockade of group II metabotropic glutamate receptors reduces the growth of glioma cells in vivo. Neuro-oncology 7:236–245
Baia GS, Dinca EB, Ozawa T, Kimura ET, McDermott MW, James CD et al (2008) An orthotopic skull base model of malignant meningioma. Brain Pathol 18:172–179
Bibby MC (2004) Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer 40:852–857
Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS et al (1990) Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res 50:8017–8022
Bolon B (2004) Genetically engineered animals in drug discovery and development: a maturing resource for toxicologic research. Basic Clin Pharmacol Toxicol 95:154–161
Burgos JS, Rosol M, Moats RA, Khankaldyyan V, Kohn DB, Nelson MD Jr et al (2003) Time course of bioluminescent signal in orthotopic and heterotopic brain tumors in nude mice. Biotechniques 34:1184–1188
Contag CH, Bachmann MH (2002) Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 4:235–260
Dinca E, Kitange G, Schroeder M, James CD (2006) Quantitative optical imaging correlates better with tumor volume for luminescent compared to fluorescent imaging in a U87 orthotopic murine model of glioblastoma multiforme. In: The 36th Annual Meeting of the Society for Neuroscience 2006, Atlanta, GA
Dinca EB, Lu KV, Sarkaria JN, Pieper RO, Prados MD, Haas-Kogan DA et al (2008) p53 Small-molecule inhibitor enhances temozolomide cytotoxic activity against intracranial glioblastoma xenografts. Cancer Res 68:10034–10039
Dinca EB, Sarkaria JN, Schroeder MA, Carlson BL, Voicu R, Gupta N et al (2007) Bioluminescence monitoring of intracranial glioblastoma xenograft: response to primary and salvage temozolomide therapy. J Neurosurg 107:610–616
Edinger M, Cao YA, Hornig YS, Jenkins DE, Verneris MR, Bachmann MH et al (2002) Advancing animal models of neoplasia through in vivo bioluminescence imaging. Eur J Cancer 38:2128–2136
Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP (1991) Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res 51:2164–2172
Fomchenko EI, Holland EC (2006) Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res 12:5288–5297
Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL et al (2005) Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncology 7:164–176
Giese A (2003) Glioma invasion—pattern of dissemination by mechanisms of invasion and surgical intervention, pattern of gene expression and its regulatory control by tumor suppressor p53 and proto-oncogene ETS-1. Acta Neurochir Suppl 88:153–162
Gross S, Piwnica-Worms D (2005) Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell 7:5–15
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Hasegawa K, Pham L, O'Connor MK, Federspiel MJ, Russell SJ, Peng KW (2006) Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res 12:1868–1875
Hashizume R, Ozawa T, Dinca EB, Banerjee A, Prados MD, James CD et al (2010) A human brainstem glioma xenograft model enabled for bioluminescence imaging. J Neurooncol 96(2):151–159
Hollingshead MG, Bonomi CA, Borgel SD, Carter JP, Shoemaker R, Melillo G et al (2004) A potential role for imaging technology in anticancer efficacy evaluations. Eur J Cancer 40:890–898
Humphrey PA, Wong AJ, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE et al (1990) Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci USA 87:4207–4211
Kadoch C, Dinca EB, Voicu R, Chen L, Nguyen D, Parikh S et al (2009) Pathologic correlates of primary central nervous system lymphoma defined in an orthotopic xenograft model. Clin Cancer Res 15:1989–1997
Kerbel RS (2003) Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol Ther 2:S134–S139
Kitange GJ, Templeton KL, Jenkins RB (2003) Recent advances in the molecular genetics of primary gliomas. Curr Opin Oncol 15:197–203
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM et al (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9:391–403
Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H et al (1985) Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 313:144–147
Lu KV, Zhu S, Cvrljevic A, Huang TT, Sarkaria S, Ahkavan D et al (2009) Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res 69:6889–6898
Malden LT, Novak U, Kaye AH, Burgess AW (1988) Selective amplification of the cytoplasmic domain of the epidermal growth factor receptor gene in glioblastoma multiforme. Cancer Res 48:2711–2714
Pandita A, Aldape KD, Zadeh G, Guha A, James CD (2004) Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosom Cancer 39:29–36
Paroo Z, Bollinger RA, Braasch DA, Richer E, Corey DR, Antich PP et al (2004) Validating bioluminescence imaging as a high-throughput, quantitative modality for assessing tumor burden. Mol Imaging 3:117–124
Ragel BT, Elam IL, Gillespie DL, Flynn JR, Kelly DA, Mabey D et al (2008) A novel model of intracranial meningioma in mice using luciferase-expressing meningioma cells. Laboratory investigation. J Neurosurg 108:304–310
Rangarajan A, Weinberg RA (2003) Opinion: comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer 3:952–959
Ray P, De A, Min JJ, Tsien RY, Gambhir SS (2004) Imaging tri-fusion multimodality reporter gene expression in living subjects. Cancer Res 64:1323–1330
Ray P, Wu AM, Gambhir SS (2003) Optical bioluminescence and positron emission tomography imaging of a novel fusion reporter gene in tumor xenografts of living mice. Cancer Res 63:1160–1165
Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH et al (2000) Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2:491–495
Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K et al (2003) A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA 100:13513–13518
Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, Schroeder MA et al (2007) Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther 6:1167–1174
Schmidt NO, Ziu M, Carrabba G, Giussani C, Bello L, Sun Y et al (2004) Antiangiogenic therapy by local intracerebral microinfusion improves treatment efficiency and survival in an orthotopic human glioblastoma model. Clin Cancer Res 10:1255–1262
Shah K, Bureau E, Kim DE, Yang K, Tang Y, Weissleder R et al (2005) Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol 57:34–41
Soling A, Rainov NG (2003) Bioluminescence imaging in vivo—application to cancer research. Expert Opin Biol Ther 3:1163–1172
Sugawa N, Ekstrand AJ, James CD, Collins VP (1990) Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci USA 87:8602–8606
Sweet-Cordero A, Mukherjee S, Subramanian A, You H, Roix JJ, Ladd-Acosta C et al (2005) An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat Genet 37:48–55
Szentirmai O, Baker CH, Lin N, Szucs S, Takahashi M, Kiryu S et al (2006) Noninvasive bioluminescence imaging of luciferase expressing intracranial U87 xenografts: correlation with magnetic resonance imaging determined tumor volume and longitudinal use in assessing tumor growth and antiangiogenic treatment effect. Neurosurgery 58:365–372, discussion -72
Tang Y, Shah K, Messerli SM, Snyder E, Breakefield X, Weissleder R (2003) In vivo tracking of neural progenitor cell migration to glioblastomas. Hum Gene Ther 14:1247–1254
Weissleder R (2002) Scaling down imaging: molecular mapping of cancer in mice. Nat Rev Cancer 2:11–18
Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V et al (2007) Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–660
Yamazaki H, Fukui Y, Ueyama Y, Tamaoki N, Kawamoto T, Taniguchi S et al (1988) Amplification of the structurally and functionally altered epidermal growth factor receptor gene (c-erbB) in human brain tumors. Mol Cell Biol 8:1816–1820
Yamini B, Yu X, Gillespie GY, Kufe DW, Weichselbaum RR (2004) Transcriptional targeting of adenovirally delivered tumor necrosis factor alpha by temozolomide in experimental glioblastoma. Cancer Res 64:6381–6384
Zhang Z, Tang LL, Zhan RY, Tong Y, Yao HP, Du LA (2004) Immunotherapy of intracranial G422 glioblastoma with dendritic cells pulsed with tumor extract or RNA. J Zhejiang Univ Sci 5:1298–1303
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Allan H. Friedman, Durham, USA
Bioluminescence imaging (BLI) of invasive intracranial xenografts: implications for translational research and targeted therapeutics of brain tumors
As the authors so well describe, we have made great progress at treating models of glioblastomas but sadly have made modest progress treating patients harboring glioblastomas. The problem is that the models do not accurately reproduce the disease. Cells grown out in cultures in media containing an artificial arrangement of growth factors are very different than cells that have developed strategies to survive in the human brain. Tumors implanted into laboratory animals are greatly influenced by the micro environment of the host tissue receiving the implant. I disagree with the author’s dismissal of the modified immune system in athymic mice. It is possible that the intrinsic immune system can become important once the tumor has been wounded by a therapeutic intervention. It is also possible that the strategies tumors use to thwart adaptive immunity are not relevant in the nascent immune system of athymic mice. Whatever the reason, it is much easier to eradicate a GBM in an athymic mouse than in a patient.
The authors describe their work and review the literature using bioluminescence to monitor the growth of intracranially implanted modified malignant xenografts. I myself have no experience with this technique, but I found the authors’ review to be quite compelling. The luciferase-transfected cells seemed to behave in a fashion similar to nontransfected cells. The transfection seemed to be stable over time in cell culture and in implanted grafts. The measured fluorescence correlated well with tumor volume as measured by in vivo MRI and histologically reconstructed tumors. The authors are correct in noting that animal MRI imaging’s equipment is expensive and somewhat cumbersome to use.
The author’s bibliography provides the interested reader with references to the transfection technique and the mechanics of measuring bioluminescence from intracranially implanted tumors. We should not lose track of the author’s point that the models of brain tumors presently available only partially replicate the human condition.
Matthias Kirsch, Dresden, Germany
The authors present a timely review of an established technology using luciferase-induced luminescence for in vivo imaging of tumor growth that still has to find its way into routine monitoring in experimental neuro-oncology.
Bioluminescence imaging is an important adjunct to the experimental armamentarium in neuro-oncology since it offers the possibility for frequent intravital measurements and quantification of tumor growth. It cannot offer morphological resolution as MRI does but is cheaper and much easier to use. BLI is limited to integral two-dimensional imaging, yet—at least in subcutaneous tumor models—bioluminescent data permitted earlier detection of tumor growth than standard caliper measurements.
Currently, it is limited due to the limited penetration depth of light and, therefore, to its principle applications in subcutaneous or intraperitoneal tumor models. However, as the current review demonstrates, experiments in small rodents with thin skulls have shown its applicability for orthotopic brain tumor investigations. Bioluminescence imaging has not reached its limits: Three-dimensional measurements using dual projections and advanced software algorithms will further improve its usability as a brain tumor monitoring device.
Ghazaleh Tabatabai, Zurich, Switzerland
The prognosis for patients with malignant gliomas has remained poor. Novel therapeutic approaches are urgently required. Before evaluating new therapeutic strategies in clinical trials, however, it is important to thoroughly examine their efficacy and toxicity in preclinical models. Today, most preclinical studies evaluating anti-glioma therapies are performed in cell culture and rodent models. Dinca and colleagues provide an overview on currently used in vitro and in vivo techniques for studying brain tumor biology. Cell culture studies either using murine or human glioma cell lines are very helpful for performing “high throughput” studies, i.e., testing different compounds or different combinations thereof in multiple parallel experiments. The main aspect of tumor biology, however, the impact of the tumor host interaction, cannot be adequately simulated in cell culture. Thus, the translational relevance of in vitro studies alone remains questionable. Clinical relevance is supported by studying the efficacy of anti-glioma therapies in mouse models. For studying orthotopic human experimental gliomas, the human glioma cells are implanted into the brains of nude mice. Migration and invasion of human glioma cells can be reliably studied in nude mice. The important aspects of immunological interactions, however, are not reflected by studies in nude mice. Therefore, some authors suggested the use of genetically modified mice by silencing a tumor suppressor gene or overexpressing an oncogene. These approaches, on the other hand, do not mimic the sporadic occurrence of malignant gliomas. Another helpful tools are syngeneic mouse models, i.e. implanting murine glioma cells into the brain of syngeneic mice, e.g., GL-261 and C57Bl/6. Taken together, even though the different mouse models have their respective limitations, it will be important for scientists to design their studies by combining different mouse models, implanting different glioma cell lines or patient-derived cells, and thus combining the strengths and reducing the limitations. Still, for assessing anti-glioma therapies in orthotopic glioma models, it will be important to use a noninvasive imaging tool for longitudinal monitoring of efficacy. In this regard, BLI and magnetic resonance imaging (MRI) are very useful. Both techniques allow close follow-up monitoring of intracranial tumor growth during therapy. Based on these studies, future preclinical assessments of anti-glioma therapies will more closely resemble clinical follow-up neuroimaging approaches in individual patients. This will hopefully further optimize preclinical assessments and enable better clinical translations.
Rights and permissions
About this article
Cite this article
Dinca, E.B., Voicu, R.V. & Ciurea, A.V. Bioluminescence imaging of invasive intracranial xenografts: implications for translational research and targeted therapeutics of brain tumors. Neurosurg Rev 33, 385–394 (2010). https://doi.org/10.1007/s10143-010-0275-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-010-0275-4