Abstract
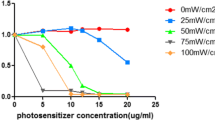
Hematoporphyrin monomethyl ether (HMME) is a novel and promising porphyrin-related photosensitizer for photodynamic therapy (PDT). This study aimed to investigate the efficacy and potential mechanism of HMME-PDT under irradiation of green light-emitting diode (LED) with wavelength of 530 ± 20 nm in treating human tongue squamous cell carcinoma Tca8113 cells in vitro. The HMME concentrations were 1.25, 2.5, and 5 μg/ml while the energy densities were 0.6, 1.2, 1.8, 2.4, and 3.0 J/cm2. MTT assay demonstrated that HMME-PDT significantly inhibited the proliferation of Tca8113 cells, and the cytotoxicity was improved with increased HMME concentration and light intensity. The amount of cells decreased significantly and the morphology of cells changed drastically after HMME-PDT. Flow cytometry analysis revealed that HMME-PDT induced both apoptosis and necrosis, but apoptosis was the main form of cell death. Apoptotic morphology was confirmed by Hoechst 33342 staining. Laser scanning confocal microscopy observation showed that HMME was mainly localized in mitochondria. The production of intracellular reactive oxygen species increased remarkably after PDT treatment, and both sodium azide (the singlet oxygen quencher) and d-mannitol (the hydroxyl radical scavenger) could protect Tca8113 cells from death induced by HMME-PDT. Additionally, the activity of caspase-3 also increased markedly in treated groups, and the cell death could be rescued by a reversible inhibitor (Ac-DEVD-CHO) of caspase-3. These results demonstrated that HMME combined with green LED significantly induced apoptosis of Tca8113 cells, suggesting that HMME-PDT using green LED might be a potential therapeutic strategy for human tongue squamous cell carcinoma.





Similar content being viewed by others
References
Neville BW, Day TA (2002) Oral cancer and precancerous lesions. CA Cancer J Clin 52:195–215
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45:309–316
Prince S, Bailey BM (1999) Squamous carcinoma of the tongue: review. Br J Oral Maxillofac Surg 37:164–174
Sharman WM, Allen CM, van Lier JE (1999) Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today 4:507–517
Dolmans DE, Fukumura D, Jain RK (2003) Photodynamic therapy for cancer. Nat Rev Cancer 3:380–387
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D et al (1998) Photodynamic therapy. J Natl Cancer Inst 90:889–905
Pervaiz S, Olivo M (2006) Art and science of photodynamic therapy. Clin Exp Pharmacol Physiol 33:551–556
Ding X, Xu Q, Liu F, Zhou P, Gu Y et al (2004) Hematoporphyrin monomethyl ether photodynamic damage on HeLa cells by means of reactive oxygen species production and cytosolic free calcium concentration elevation. Cancer Lett 216:43–54
Song K, Kong B, Qu X, Li L, Yang Q (2005) Phototoxicity of hemoporfin to ovarian cancer. Biochem Biophys Res Commun 337:127–132
Yuan KH, Li Q, Yu WL, Zeng D, Zhang C et al (2008) Comparison of photodynamic therapy and pulsed dye laser in patients with port wine stain birthmarks: a retrospective analysis. Photodiagnosis Photodyn Ther 5:50–57
Shi J, Ma R, Wang L, Zhang J, Liu R et al (2013) The application of hyaluronic acid-derivatized carbon nanotubes in hematoporphyrin monomethyl ether-based photodynamic therapy for in vivo and in vitro cancer treatment. Int J Nanomedicine 8:2361–2373
Zeng H, Sun M, Zhou C, Yin F, Wang Z et al (2013) Hematoporphyrin monomethyl ether-mediated photodynamic therapy selectively kills sarcomas by inducing apoptosis. PLoS One 8, e77727
Brancaleon L, Moseley H (2002) Laser and non-laser light sources for photodynamic therapy. Lasers Med Sci 17:173–186
Juzeniene A, Juzenas P, Ma LW, Iani V, Moan J (2004) Effectiveness of different light sources for 5-aminolevulinic acid photodynamic therapy. Lasers Med Sci 19:139–149
Liu Y, Ma XQ, Jin P, Li HT, Zhang RR et al (2011) Apoptosis induced by hematoporphyrin monomethyl ether combined with He-Ne laser irradiation in vitro on canine breast cancer cells. Vet J 188:325–330
Zhan Q, Yue W, Hu S (2011) Effect of photodynamic therapy and endostatin on human glioma xenografts in nude mice. Photodiagn Photodyn Ther 8:314–320
Li BH, Xie SS, Lu ZK (2002) Spectral properties of new photosensitizers for photodynamic diagnosis and therapy. Guang Pu Xue Yu Guang Pu Fen Xi 22:902–904
Xu H, Liu C, Mei J, Yao C, Wang S et al (2012) Effects of light irradiation upon photodynamic therapy based on 5-aminolevulinic acid-gold nanoparticle conjugates in K562 cells via singlet oxygen generation. Int J Nanomedicine 7:5029–5038
Hino H, Murayama Y, Nakanishi M, Inoue K, Nakajima M et al (2013) 5-Aminolevulinic acid-mediated photodynamic therapy using light-emitting diodes of different wavelengths in a mouse model of peritoneally disseminated gastric cancer. J Surg Res 185:119–126
Moseley H (1996) Total effective fluence: a useful concept in photodynamic therapy. Lasers Med Sci 11:139–143
Agostinis P, Buytaert E, Breyssens H, Hendrickx N (2004) Regulatory pathways in photodynamic therapy induced apoptosis. Photochem Photobiol Sci 3:721–729
Wyld L, Reed MW, Brown NJ (2001) Differential cell death response to photodynamic therapy is dependent on dose and cell type. Br J Cancer 84:1384–1386
Ahmad N, Mukhtar H (2000) Mechanism of photodynamic therapy-induced cell death. Methods Enzymol 319:342–358
Dahle J, Steen HB, Moan J (1999) The mode of cell death induced by photodynamic treatment depends on cell density. Photochem Photobiol 70:363–367
Peng Q, Moan J, Nesland JM (1996) Correlation of subcellular and intratumoral photosensitizer localization with ultrastructural features after photodynamic therapy. Ultrastruct Pathol 20:109–129
Morgan J, Oseroff AR (2001) Mitochondria-based photodynamic anti-cancer therapy. Adv Drug Deliv Rev 49:71–86
Kessel D, Luo Y, Deng Y, Chang CK (1997) The role of subcellular localization in initiation of apoptosis by photodynamic therapy. Photochem Photobiol 65:422–426
Hsieh YJ, Wu CC, Chang CJ, Yu JS (2003) Subcellular localization of Photofrin determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy: when plasma membranes are the main targets. J Cell Physiol 194:363–375
Song K, Kong B, Li L, Yang Q, Wei Y et al (2007) Intraperitoneal photodynamic therapy for an ovarian cancer ascite model in Fischer 344 rat using hematoporphyrin monomethyl ether. Cancer Sci 98:1959–1964
Ito K, Nakazato T, Yamato K, Miyakawa Y, Yamada T et al (2004) Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res 64:1071–1078
Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J et al (2005) Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem 280:19911–19924
Oleinick NL, Evans HH (1998) The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res 150:S146–S156
Lawen A (2003) Apoptosis-an introduction. Bioessays 25:888–896
Liu T, Wu LY, Choi JK, Berkman CE (2010) Targeted photodynamic therapy for prostate cancer: inducing apoptosis via activation of the caspase-8/-3 cascade pathway. Int J Oncol 36:777–784
Cheng J, Liang H, Li Q, Peng C, Li Z et al (2010) Hematoporphyrin monomethyl ether-mediated photodynamic effects on THP-1 cell-derived macrophages. J Photochem Photobiol B 101:9–15
Acknowledgments
The authors would like to thank Dr. Juan Wang for her kindly providing the human TSCC Tca8113 cell line.
Conflict of interest
The authors declare that there is no conflict of interest regarding this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xingqiang Lai and Fen Ning contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lai, X., Ning, F., Xia, X. et al. HMME combined with green light-emitting diode irradiation results in efficient apoptosis on human tongue squamous cell carcinoma. Lasers Med Sci 30, 1941–1948 (2015). https://doi.org/10.1007/s10103-015-1774-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1774-x