Abstract
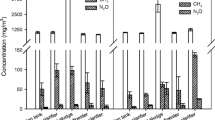
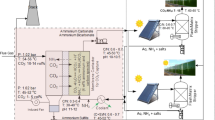
Carbon dioxide absorption using residual sludge rich in ammonia as absorbent is studied. Ammonia concentrations in residual sludge rich in ammonia are at low concentrations (<0.2 wt%) compared to ammonia concentrations industrially used for carbon dioxide absorption solvents (8–10 wt%). The proposed solution avoids emissions of ammonia and carbon dioxide at the same time. The resulting aqueous stream is useful for fertigation, and therefore, energy consumption for solvent recovery by absorption is not required. The stability of the output stream is checked. The relation between minimum required solvent flow rate, solvent concentration and C:N ratio is determined. Minimum flow rate of solvent is defined as the lowest solvent flowrate required to satisfy the separation problem, using infinite length absorption column. However, due to the low ammonia content required for a residual sludge with a C:N ratio suitable for algae growth (mass fractions from 0.000182 to 0.000063), a high flow rate of absorbent is still required (only from 10 to 20 % flow rate decreases compared with pure water). Nevertheless, C:N ratios around 1 lead to a decrease of solvent flow rate requirements of 85.3 % compared to pure water solvent. Although this ratio is not suitable for plant growth, terrestrial plants capture the carbon dioxide mainly from the atmosphere equilibrating the balance. An illustrative example is presented to show that irrigation water can become a suitable sink for carbon dioxide and ammonia, providing fertigation. Therefore, two residual streams (sludge and carbon dioxide) are converted into a useful fertigation stream which is of great importance because most of the water that humanity consumes is not only for drinking but for food production as well. Nowadays, ammonia studies on carbon dioxide absorption are controlled by the physical absorption (0 % NH3) or by the chemical absorption (8–10 % NH3), simplifying the modelling. The obtained results show the importance of the study and identification of the region where physical and chemical absorptions are both significant.










Similar content being viewed by others
References
Allen RG, Pereira LS, Raes D, Smith M. (1998) Crop evapotranspiration-guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. ISBN 92-5-104219-5. FAO-Food and Agriculture Organization of the United Nations.www.fao.org/docrep/x0490e/x0490e00.HTM. Accessed 20 Dec 2014
Altman I, Hunter AM (2014) The employment and income effects of cleaner coal: the case of FutureGen and rural Illinois. Clean Technol Environ Policy. doi:10.1007/s10098-014-0872-y
Bandyopadhyay A (2011) Amine versus ammonia absorption of CO2 as a measure of reducing GHG emission: a critical analysis. Clean Technol Environ Policy 13(2):269–294
Barbosa D, Doherty MF (1987) New set of composition variables for the representation of reactive-phase diagrams. Proc R Soc Lond A 413:459–464
BOE (2014) boe.es/boe/dias/2014/11/24/pdfs/BOE-A-2014-12190.pdf. Accessed 20 Dec 2014
Brown D, Sadiq R, Hewage K (2014) An overview of air emission intensities and environmental performance of grey cement manufacturing in Canada. Clean Technol Environ Policy 16(6):1119–1131
Budzianowski WM (2011) Mitigating NH3 vaporization from an aqueous ammonia process for CO2 capture. Int J Chem Reactor Eng 9:A58
Canalsurgell (2014) Canalsurgell.org/historia/el-canal/. Accessed 20 Dec 2014
Chong FK, Eljack FT, Atilhan M, Foo DCY, Chemmangattuvalappil NG (2014) Ionic liquid design for enhanced carbon dioxide capture–a computer aided molecular design approach. Chem Eng Trans 39:253–258
Cormos CC (2014) Techno-economic and environmental analysis of hydrogen and power co-generation based on co-gasification of coal and biomass/solid wastes with carbon capture. Chem Eng Trans 37:139–144
Dave N, Do T, Puxty G, Rowland R, Feron PHM, Attalla MI (2009) CO2 capture by aqueous amines and aqueous ammonia-a comparison. Energy Proced 1(1):949–954
Egea G, González-Real MM, Martin-Gorriz B, Baille A (2014) Leaf-to-branch scaling of C-gain in field-grown almond trees under different soil moisture regimes. Tree Physiol 34(6):619–629
FAO AQUASTAT (2014) fao.org/nr/water/aquastat/water_use/index.stm. Accessed 20 Dec 2014
Fong LY, Anderson C, Hooper B, Xiao G, Webley PA, Hoadley A (2014) Multi-objective optimisation of hybrid CO2 capture processes using exergy analysis. Chem Eng Trans 39:1501–1506
Gong J, You F (2014) Biological carbon sequestration and utilization via algal biorefinery. Chem Eng Trans 39:217–222 (Special Issue)
Gross A, Guy O, Posmanik R, Fine P, Nejidat A (2012) A novel method for combined biowaste stabilization and production of nitrate-rich liquid fertilizer for use in organic horticulture. Water Air Soil Pollut 223(3):1205–1214
Hasaneen R, Elsayed NA, Barrufet MA (2014) Analysis of the technical, microeconomic, and political impact of a carbon tax on carbon dioxide sequestration resulting from liquefied natural gas production. Clean Technol Environ Policy. doi:10.1007/s10098-014-0735-6
Klemeš J, Bulatov I, Cockerill T (2007) Techno-economic modelling and cost functions of CO2 capture processes. Comput Chem Eng 31(5–6):445–455
Mackinnon IDR, Barr K, Miller E, Hunter S, Pinel T (2003) Nutrient removal from wastewaters using high performance materials. Water Sci Technol 47:101–107
Makhloufi C, Lasseugette E, Remigy JC, Belaissaoui B, Roizard D, Favre E (2014) Ammonia based CO2 capture process using hollow fiber membrane contactors. J Membr Sci 455:236–246
Mani F, Peruzzini M, Stoppioni P (2006) CO2 absorption by aqueous NH3 solutions: speciation of ammonium carbamate, bicarbonate and carbonate by a 13C NMR study. Green Chem 8:995–1000
McLeod A, Jefferson B, McAdam EJ (2014) Biogas upgrading by chemical absorption using ammonia rich absorbents derived from wastewater. Water Res 67:175–186
Mendes L, De Medeiros JL, Alves RMB, Araújo OQF (2014) Production of methanol and organic carbonates for chemical sequestration of CO2 from an NGCC power plant. Clean Technol Environ Policy 16(6):1095–1105
Meng X, Luo D (2013) An evaluation method of CO2-EOR social benefit for authoritative incentive policy-making. Clean Technol Environ Policy 15(6):1083–1089
Miles DH, Wilson GM (1975) Vapor liquid equilibrium data for design of sour water strippers. Annual report to the API for 1974. American Petroleum Institute (API), Washington
Mingaleeva GR, Nikolaev AN, Shamsutdinov EV (2014) Utilization of emissions of CO2 by production of mineral fertilizers. Chem Eng Trans 39:1117–1122
Murtić S, Čmivić H, Durić M, Paunovic G, Sekularac G, Behmen F, Krsmanović M (2013) The content of some antioxidants in apple depends on the type of fertilization. Pol J Environ Stud 22(2):475–480
Perry S, Klemeš J, Bulatov I (2008) Integrating waste and renewable energy to reduce the carbon footprint of locally integrated energy sectors. Energy 33(10):1489–1497
Phuntsho S, Shon HK, Hong S, Lee S, Vigneswaran S, Kandasamy J (2012) Fertiliser drawn forward osmosis desalination: the concept, performance and limitations for fertigation. Rev Environ Sci Biotechnol 11(2):147–168
Pinsent BRW, Pearson L, Roughton FJW (1956) The kinetics of combination of carbon dioxide with ammonia. Trans Faraday Soc 52:1594–1598
Puerari F, Bosio B, Heyen G (2014) Energy efficiency optimisation in different plant solutions for methanol production from biomass gasification. Chem Eng Trans 37:301–306
Rao AB, Rubin E (2002) A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ Sci Technol 36:4467–4475
Rashidi NA, Yusup S, Borhan A, Loong LH (2014) Experimental and modelling studies of carbon dioxide adsorption by porous biomass derived activated carbon. Clean Technol Environ Policy. doi:10.1007/s10098-014-0788-6
RCCDE (2013) canviclimatic.gencat.cat/web/.content/home/comerc_de_drets_demissio/resultat_del_seguiment_demissions/periode_2013/resum_emissions_rccde_2013_web_28032014.pdf. Accessed 20 Dec 2014
Russell DL (2006) Practical wastewater treatment. John Wiley & Sons Inc, Hoboken
Sala M, Bonet J, Plesu V, Bonet-Ruiz AE, Iancu P, Llorens J (2014) Minimum gas/liquid flow rate for absorption columns. Chem Eng Trans 39:241–246
Schiavon Maia DC, Cardozo F, Frare L, Gimenes M, Pereira N (2014) Purification of biogas for energy use. Chem Eng Trans 37:643–648
Singh S, Mukherjee C, Verma A (2014) Development of catalytic activity protocol for electrochemical reduction of carbon dioxide to value added products. Clean Technol Environ Policy. doi:10.1007/s10098-014-0796-6
Spirov P, Rudyk S, Tyrovolas A, Jimoh I (2013) The bitumen extraction from Nigerian tar sand using dense carbon dioxide. Chem Eng Trans 32:283–288
Thornton A, Pearce P, Parsons SA (2007) Ammonium removal from digested sludge liquors using ion exchange. Water Res 41:433–439
UNWATER (2014) unwater.org/statistics/statistics-detail/en/c/211408/. Accessed 20 Dec 2014
Varbanov P, Klemeš J (2008) Analysis and integration of fuel cell combined cycles for development of low-carbon energy technologies. Energy 33(10):1508–1517
Yang DX, Zeng RS, Zhang Y, Wang ZQ, Wang S, Jin C (2012) Numerical simulation of multiphase flows of CO2 storage in saline aquifers in Daqingzijing oilfield China. Clean Technol Environ Policy 14(4):609–618
Yang N, Yu H, Li L, Xu D, Han W, Feron P (2014) Aqueous ammonia (NH3) based post combustion CO2 capture: a review. Oil Gas Sci Technol 69(5):931–945
Zhao B, Su Y, Liu W (2015) Publication-based survey for status of scientific research and impact on post-combustion CO2 capture. Int J Greenh Gas Control 32:56–60
Acknowledgments
The authors would like to thank the financial support of POSCCE project ID 652 (Structural Funds for Development and Cohesion).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonet-Ruiz, AE., Plesu, V., Bonet, J. et al. Preliminary technical feasibility analysis of carbon dioxide absorption by ecological residual solvents rich in ammonia to be used in fertigation. Clean Techn Environ Policy 17, 1313–1321 (2015). https://doi.org/10.1007/s10098-015-0950-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-015-0950-9