Abstract
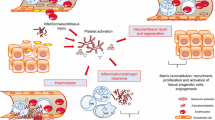
Platelet activation mediates systemic inflammatory response during infection. However, data on platelet reactivity (PR) varies among different settings. We assessed PR along different stages of sepsis and tried to predict for determinants of its variance. In parallel, we evaluated it as an early bedside diagnostic biomarker. This was an observational prospective cohort study. Incoming patients were assorted to distinct groups of uncomplicated infection, sepsis, and severe sepsis/septic shock. A control group of healthy volunteers was used as comparison. PR was assessed using the bedside point-of-care VerifyNow assay, in P2Y12 reaction units (PRU) alongside with levels of major inflammatory markers and whole blood parameters. A total of 101 patients and 27 healthy volunteers were enrolled. PR significantly and reversibly increases during sepsis compared to uncomplicated infection and healthy controls (244 ± 66.7 vs 187.33 ± 60.98, p < 0.001 and 192.17 ± 47.51, p < 0.001, respectively). In severe sepsis, PR did not significantly differ compared to other groups. Sepsis stage uniquely accounts for 15.5% of PR in a linear regression prediction model accounting for 30% of the variance of PR (F = 8.836, p < 0.001). PRU >253 had specificity of 91.2% and sensitivity of 40.8% in discriminating septic from non-septic patients. The addition of PRU to SOFA and qSOFA scores significantly increased their c-statistic (AUC SOFA + PRU, 0.867 vs SOFA, 0.824, p < 0.003 and AUC qSOFA + PRU, 0.842 vs qSOFA, 0.739, p < 0.001), making them comparable (AUC SOFA + PRU vs qSOFA + PRU, p = 0.4). PR significantly and reversibly increases early in sepsis, but seems to exhaust while disease progresses. Bedside assessment of PR can provide robust discriminative accuracy in the early diagnosis of septic patients.



Similar content being viewed by others
References
Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, Tracey K, van der Poll T, Pelfrene E (2015) Sepsis: a roadmap for future research. Lancet Infect Dis 15(5):581–614
Gogos CA, Drosou E, Bassaris HP, Skoutelis A (2000) Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181(1):176–180
Hotchkiss RS, Monneret G, Payen D (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13(12):862–874
Novosad S, Sapiano M, Grigg C, LakeJ, Robyn M, Dumyati G, Felsen C, Blog D, Dufort E, Zansky S, Wiedeman K, Avery l, Dantes R, Jernigan J, Magill S, Fiore A, Epstein L (2016) Vital Signs: Epidemiology of Sepsis: Prevalence of Health Care Factors and Opportunities for Prevention. MMWR Morb Mortal Wkly Rep 65(33):864–869
Thomas MR, Storey RF (2015) The role of platelets in inflammation. Thromb Haemost 114(3):449–458
Akinosoglou K, Alexopoulos D (2014) Use of antiplatelet agents in sepsis: a glimpse into the future. Thromb Res 133(2):131–138
Liverani E, Rico MC, Tsygankov AY, Kilpatrick LE, Kunapuli SP (2016) P2Y12 receptor modulates sepsis-induced inflammation. Arterioscler Thromb Vasc Biol 36(5):961–971
Liverani E, Rico MC, Yaratha L, Tsygankov AY, Kilpatrick LE, Kunapuli SP (2014) LPS-induced systemic inflammation is more severe in P2Y12 null mice. J Leukoc Biol 95(2):313–323
Evangelista V, dell’Elba G, Martelli N, Amore C, Pecce R,Piccoli A, Manarini S, Totani L (2007) Anti-inflammatory effects of clopidogrel in the mouse. J Thromb Haemost 5 (Suppl 2):P-M-283
Winning J, Baranyai J, Claus R, Eisenhut I, Hamacher J, Reinhart K, Bauer M, Lösche W (2007) Beneficial effects of antiplatelet drugs in patients with community-acquired pneumonia and in endotoxin shock in mice. Crit Care 11(Suppl 2):P27
Thomas MR, Outteridge SN, Ajjan RA, Phoenix F, Sangha GK, Faulkner RE, Ecob R, Judge HM, Khan H, West LE, Dockrell DH, Sabroe I, Storey RF (2015) Platelet P2Y12 inhibitors reduce systemic inflammation and its Prothrombotic effects in an experimental human model. Arterioscler Thromb Vasc Biol 35(12):2562–2570
Storey RF, James SK, Siegbahn A, Varenhorst C, Held C, Ycas J, Husted SE, Cannon CP, Becker RC, Steg PG, Asenblad N, Wallentin L (2014) Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets 25(7):517–525
Schoergenhofer C, Schwameis M, Hobl EL, Ay C, Key NS, Derhaschnig U, Jilma B, Spiel AO (2016) Potent irreversible P2Y12 inhibition does not reduce LPS-induced coagulation activation in a randomized, double-blind, placebo-controlled trial. Clin Sci 130(6):433–440
von Beckerath N, Pogatsa-Murray G, Wieczorek A, Sibbing D, Schomig A, Kastrati A (2006) Correlation of a new point-of-care test with conventional optical aggregometry for the assessment of clopidogrel responsiveness. Thromb Haemost 95(5):910–911
Boldt J, Menges T, Wollbruck M, Sonneborn S, Hempelmann G (1994) Platelet function in critically ill patients. Chest 106(3):899–903
Gawaz M, Dickfeld T, Bogner C, Fateh-Moghadam S, Neumann FJ (1997) Platelet function in septic multiple organ dysfunction syndrome. Intensive Care Med 23(4):379–385
Johansson D, Shannon O, Rasmussen M (2011) Platelet and neutrophil responses to gram positive pathogens in patients with bacteremic infection. PLoS One 6(11):e26928
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31(4):1250–1256
Akinosoglou K, Perperis A, Theodoraki S, Alexopoulos D, Gogos C (2017) Sepsis favors high-on-clopidogrel platelet reactivity. Platelets 21:1–3
Corrales-Medina VF, Suh KN, Rose G, Chirinos JA, Doucette S, Cameron DW, Fergusson DA (2011) Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med 8(6):e1001048
Dalager-Pedersen M, Sogaard M, Schonheyder HC, Nielsen H, Thomsen RW (2014) Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation 129(13):1387–1396
Leytin V, Shakoor S, Mody M, Allen D, Garvey B, Freedman J (2002) Sepsis- and endotoxemia-generated cytokines do not trigger activation of human platelets. Crit Care Med 30(12):2771–2773
Davies GR, Mills GM, Lawrence M, Battle C, Morris K, Hawkins K, Williams PR, Davidson S, Thomas D, Evans PA (2014) The role of whole blood impedance aggregometry and its utilisation in the diagnosis and prognosis of patients with systemic inflammatory response syndrome and sepsis in acute critical illness. PLoS One 9(9):e108589
Sjovall F, Morota S, Asander Frostner E, Hansson MJ, Elmer E (2014) Cytokine and nitric oxide levels in patients with sepsis--temporal evolvement and relation to platelet mitochondrial respiratory function. PLoS One 9(5):e97673
Mavrommatis AC, Theodoridis T, Orfanidou A, Roussos C, Christopoulou-Kokkinou V, Zakynthinos S (2000) Coagulation system and platelets are fully activated in uncomplicated sepsis. Crit Care Med 28(2):451–457
Alt E, Amann-Vesti BR, Madl C, Funk G, Koppensteiner R (2004) Platelet aggregation and blood rheology in severe sepsis/septic shock: relation to the sepsis-related organ failure assessment (SOFA) score. Clin Hemorheol Microcirc 30(2):107–115
Yaguchi A, Pradier O, Lobo F et al (2001) Platelet aggregation is impaired at the level of cyclooxygenase and thromboxane synthetase in severe sepsis. Intensive Care Med 27:S166
Woth G, Varga A, Ghosh S, Krupp M, Kiss T, Bogar L, Muhl D (2011) Platelet aggregation in severe sepsis. J Thromb Thrombolysis 31(1):6–12
Adamzik M, Gorlinger K, Peters J, Hartmann M (2012) Whole blood impedance aggregometry as a biomarker for the diagnosis and prognosis of severe sepsis. Crit Care 16(5):R204
Brenner T, Schmidt K, Delang M, Mehrabi A, Bruckner T, Lichtenstern C, Martin E, Weigand MA, Hofer S (2012) Viscoelastic and aggregometric point-of-care testing in patients with septic shock—cross-links between inflammation and haemostasis. Acta Anaesthesiol Scand 56(10):1277–1290
Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K (1995) Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Investig 25(11):843–851
Kirschenbaum LA, Aziz M, Astiz ME, Saha DC, Rackow EC (2000) Influence of rheologic changes and platelet-neutrophil interactions on cell filtration in sepsis. Am J Respir Crit Care Med 161(5):1602–1607
Vincent JL, Yagushi A, Pradier O (2002) Platelet function in sepsis. Crit Care Med 30(5 Suppl):S313–S317
Mangalpally KK, Siqueiros-Garcia A, Vaduganathan M, Dong JF, Kleiman NS, Guthikonda S (2010) Platelet activation patterns in platelet size sub-populations: differential responses to aspirin in vitro. J Thromb Thrombolysis 30(3):251–262
Kumar V, Sharma A (2009) Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol 616(1–3):7–15
Gaddnas F, Koskela M, Koivukangas V, Risteli J, Oikarinen A, Laurila J, Saarnio J, Ala-Kokko T (2009) Markers of collagen synthesis and degradation are increased in serum in severe sepsis: a longitudinal study of 44 patients. Crit Care 13(2):R53
Baurand A, Eckly A, Bari N, Leon C, Hechler B, Cazenave JP, Gachet C (2000) Desensitization of the platelet aggregation response to ADP: differential down-regulation of the P2Y1 and P2cyc receptors. Thromb Haemost 84(3):484–491
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8):801–810
Williams JM, Greenslade JH, McKenzie JV, Chu K, Brown AF, Lipman J (2017) Systemic inflammatory response syndrome, quick sequential organ function assessment, and organ dysfunction: insights from a prospective database of ED patients with infection. Chest 151(3):586–596
Dofferhoff A, Buys J (1995) Effects of antibiotics on cytokine release. In: Vincent J (ed) Yearbook of intensive care and emergency medicine. Springer-Verlag, Berlin, pp 465-472
Gachet C (2012) P2Y(12) receptors in platelets and other hematopoietic and non-hematopoietic cells. Purinergic Signal 8(3):609–619
Matera C, Falzarano C, Berrino L, Rossi F (1992) Effects of tetanus toxin, salmonella typhimurium porin, and bacterial lipopolysaccharide on platelet aggregation. J Med 23(5):327–338
Author information
Authors and Affiliations
Contributions
ΚΑ designed the study, analyzed data, and wrote the manuscript, ST collected samples, IX analyzed the data, AP performed PR measurements, TG and AP performed blood parameter and cytokine measurements, EGB oversaw cytokine measurements and critically corrected the manuscript, and CAG oversaw the study and critically corrected the manuscript.
Funding
This work was funded by Hellenic Institute for the Study of Sepsis
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study was carried out in accordance with the ethical guidelines of the 2003 Declaration of Helsinki and we obtained the permission of the Regional Research Ethical Committee of the University General Hospital of Patras (24,958/19–12-2013). All patients provided a written informed consent while in the case of a consciousness disorder the latter was obtained from the next of kin as per national law.
Consent for publication
Written informed consent was also comprised of consent for consequent publication of patient data according to good clinical research practice.
Conflict of interest
None of the authors have a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding).
Electronic supplementary material
ESM 1
(DOC 31 kb)
ESM 2
(DOC 39 kb)
ESM 3
(DOC 55 kb)
Supplementary Figure1
A-D Respective whole blood parameter levels along different stages of sepsis (GIF 195 kb)
Rights and permissions
About this article
Cite this article
Akinosoglou, K., Theodoraki, S., Xanthopoulou, I. et al. Platelet reactivity in sepsis syndrome: results from the PRESS study. Eur J Clin Microbiol Infect Dis 36, 2503–2512 (2017). https://doi.org/10.1007/s10096-017-3093-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-3093-6