Abstract
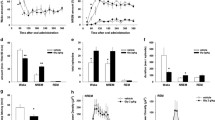
The mechanisms underlying the unconsciousness of general anesthesia are not completely understood. Accumulating evidence indicates the ventrolateral preoptic nucleus (VLPO) in the endogenous sleep circuits may contribute to loss of consciousness (LOC) induced by GABA-enhancing anesthetics. However, there are few studies that look into distinct sleep pathway in the sleep–wake system. In the neural pathway from VLPO to the locus coeruleus (LC), we compared the inhibition effect of propofol on the LC activity before and after VLPO lesion in vivo rats. Systemic administration of propofol (20 mg/kg, i.p.) in normal rats caused a fast and obvious inhibition of LC neurons spontaneous firing (from 0.24 ± 0.06 to 0.12 ± 0.03 Hz). The LC neuronal firing rate of VLPO lesion rats only decreased to 0.18 ± 0.05 Hz (P = 0.021 vs. non-VLPO rats) after the propofol injection, and the time to reach the maximal inhibition level was also prolonged in VLPO lesion rats (2.3 ± 0.7 vs. 5.8 ± 1.2 min, P = 0.037). Microinjections of a selective GABAA receptor antagonist (SR95531) into the LC fully reversed the inhibitory effect of propofol on the LC neuronal activity, but did not significantly affect the latency to loss of righting reflex of rats after propofol administration (3.4 ± 0.9 vs. 3.7 ± 1.2 min, P = 0.639). Our results indicated that VLPO is necessary for the propofol-induced inhibition of LC activity, but the LC may not play an important role in the propofol-induced LOC.






Similar content being viewed by others
References
Siegwart R, Jurd R, Rudolph U (2002) Molecular determinants for the action of general anesthetics at recombinant alpha(2)beta(3)gamma(2)gamma-aminobutyric acid(A) receptors. J Neurochem 80:140–148
Tung A, Szafran MJ, Bluhm B, Mendelson WB (2002) Sleep deprivation potentiates the onset and duration of loss of righting reflex induced by propofol and isoflurane. Anesthesiology 97:906–911
Laalou FZ, de Vasconcelos AP, Oberling P, Jeltsch H, Cassel JC et al (2008) Involvement of the basal cholinergic forebrain in the mediation of general (propofol) anesthesia. Anesthesiology 108:888–896
Lydic R, Baghdoyan HA (2005) Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology 103:1268–1295
Sherin JE, Shiromani PJ, McCarley RW, Saper CB (1996) Activation of ventrolateral preoptic neurons during sleep. Science 271:216–219
Steininger TL, Gong H, McGinty D, Szymusiak R (2001) Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol 429:638–653
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW (2012) Control of sleep and wakefulness. Physiol Rev 92:1087–1187
Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP et al (2002) The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci 5:979–984
Lu J, Nelson LE, Franks N, Maze M, Chamberlin NL et al (2008) Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol 508:648–662
Nelson LE, Lu J, Guo T, Saper CB, Franks NP et al (2003) The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 98:428–436
Eikermann M, Vetrivelan R, Grosse-Sundrup M, Henry ME, Hoffmann U et al (2011) The ventrolateral preoptic nucleus is not required for isoflurane general anesthesia. Brain Res 1426:30–37
Liu YW, Zuo W, Ye JH (2013) Propofol stimulates noradrenalin-inhibited neurons in the ventrolateral preoptic nucleus by reducing GABAergic inhibition. Anesth Analg 117:358–363
Li KY, Guan YZ, Krnjevic K, Ye JH (2009) Propofol facilitates glutamatergic transmission to neurons of the ventrolateral preoptic nucleus. Anesthesiology 111:1271–1278
Franks NP (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9:370–386
McCormick DA, Pape HC (1990) Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol 431:319–342
Brown RE, Sergeeva OA, Eriksson KS, Haas HL (2002) Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci 22:8850–8859
Fort P, Khateb A, Pegna A, Muhlethaler M, Jones BE (1995) Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea-pig brain. Eur J Neurosci 7:1502–1511
Mateo Y, Meana JJ (1999) Determination of the somatodendritic alpha2-adrenoceptor subtype located in rat locus coeruleus that modulates cortical noradrenaline release in vivo. Eur J Pharmacol 379:53–57
Correa-Sales C, Rabin BC, Maze M (1992) A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology 76:948–952
Sirois JE, Lei Q, Talley EM, Lynch C 3rd, Bayliss DA (2000) The TASK-1 two-pore domain K + channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci 20:6347–6354
Vazey EM, Aston-Jones G (2014) Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci USA 111:3859–3864
Chen CL, Yang YR, Chiu TH (1999) Activation of rat locus coeruleus neuron GABA(A) receptors by propofol and its potentiation by pentobarbital or alphaxalone. Eur J Pharmacol 386:201–210
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81460219).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Y., Yu, T., Yuan, J. et al. The ventrolateral preoptic nucleus is required for propofol-induced inhibition of locus coeruleus neuronal activity. Neurol Sci 36, 2177–2184 (2015). https://doi.org/10.1007/s10072-015-2292-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2292-0