Abstract
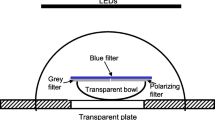
Cuttlefish are sensitive to linear polarization of light, a sensitivity that they use in predation and possibly in intraspecific communication. It has also been shown that cuttlefish are able to solve a maze using visual landmarks. In this study, cuttlefish were trained to solve a Y-maze with the e-vector of a polarized light and landmarks as redundant spatial information. The results showed that cuttlefish can use the e-vector orientation and landmarks in parallel to orient and that they are able to use either type of cue when the other one is missing. When they faced conflicting spatial information in the experimental apparatus, the majority of cuttlefish followed the e-vector rather than landmarks. Differences in response latencies in the different conditions of testing (training with both types of cue, tests with single cue or with conflicting information) were observed and discussed in terms of decision making. The ability to use near field and far field information may enable animals to interpret the partially occluded underwater light field.



Similar content being viewed by others
References
Alves C, Chichery R, Boal JG, Dickel L (2007) Orientation in the cuttlefish Sepia officinalis: response versus place learning. Anim Cogn 10:29–36. doi:10.1007/s10071-006-0027-6
Boal JG, Shashar N, Grable M, Vaughan K, Loew E, Hanlon RT (2004) Behavioral evidence for intraspecific signaling with achromatic and polarized light by cuttlefish (Mollusca: Cephalopoda). Behaviour 141:837–861. doi:10.1163/1568539042265662
Darmaillacq A-S, Chichery R, Poirier R, Dickel L (2004) Effect of early feeding experience on subsequent prey preference by cuttlefish, Sepia officinalis. Dev Psychobiol 45:239–244. doi:10.1002/dev.20034
Darmaillacq A-S, Chichery R, Shashar N, Dickel L (2006) Early familiarization overrides innate prey preference in newly hatched Sepia officinalis cuttlefish. Anim Behav 71:511–514. doi:10.1016/j.anbehav.2005.04.019
Dickel L, Boal JG, Budelmann BU (2000) The effect of early experience on learning and memory in cuttlefish. Dev Psychobiol 36:101–110
Diez-Chamizo V, Sterio D, Mackintosh NJ (1985) Blocking and overshadowing between intra-maze and extra-maze cues: a test of the independence of locale and guidance learning. Q J Exp Psychol B 37:235–253
Gallistel CR (1990) The organization of learning. The MIT Press, Cambridge
Gibson BM, Shettleworth SJ (2003) Competition among spatial cues in a naturalistic food-carrying task. Anim Learn Behav 31:143–159
Gibson BM, Shettleworth SJ (2005) Place versus response learning revisited: tests of blocking on the radial maze. Behav Neurosci 119:567–586. doi:10.3758/BF03195977
Healy S (1998) Spatial representation in animals. Oxford University Press, Oxford
Horvath G, Varju D (2004) Polarized light in animal vision: polarization patterns in nature. Springer, Berlin
Jander R, Waterman TH (1960) Sensory discrimination between polarized light and light intensity patterns by arthropods. J Cell Comp Physiol 56:137–159. doi:10.1002/jcp.1030560304
Jander R, Daumer K, Waterman TH (1963) Polarized light orientation by two Hawaiian decapod cephalopods. Z Vgl Physiol 46:383–394. doi:10.1007/BF00340466
Jozet-Alves C, Moderan J, Dickel L (2008) Sex differences in spatial cognition in an invertebrate: the cuttlefish. Proc R Soc B 275:2049–2054. doi:10.1037/0735-7044.119.2.567
Kraft P, Evangelista C, Dacke M, Labhart T, Srinivasan MV (2011) Honeybee navigation: following routes using polarized-light cues. Phil Trans R Soc B 366:703–708
Lavenex P, Schenk F (1996) Integration of olfactory information in a spatial representation enabling accurate arm choice in the radial arm maze. Learn Mem 2:299–319. doi:10.1101/lm.2.6.299
Lerner A, Sabbah S, Erlick C, Shashar N (2011) Navigation by light polarization in clear and turbid waters. Phil Trans R Soc B 366:671–679. doi:10.1098/rstb.2010.0189
Lohmann KJ, Lohmann CMF, Endres CS (2008) The sensory ecology of ocean navigation. J Exp Biol 211:1719–1728. doi:10.1242/jeb.015792
Luschi P, Seppia CD, Crosio E (1997) Orientation during short-range feeding in the crab Dotilla wichmanni. J Comp Physiol A 181:461–468
March J, Chamizo VD, Mackintosh NJ (1992) Reciprocal overshadowing between intra-maze and extra-maze cues. Q J Exp Psychol B 45:49–63
Mäthger LM, Barbosa A, Miner S, Hanlon RT (2006) Color blindness and contrast perception in cuttlefish (Sepia officinalis) determined by a visual sensorimotor assay. Vision Res 46:1746–1753
Messenger JB (1973) Learning in the cuttlefish, Sepia. Anim Behav 21:801–826
Nippak PM, Milgram MW (2005) An investigation of the relationship between response latency across several cognitive tasks in the beagle dog. Prog Neuropsychopharmacol Biol Psychiatry 29:371–377
Odling-Smee L, Braithwaite VA (2003) The role of learning in fish orientation. Fish Fish 4:235–246
Parkyn DC, Austin JD, Hawryshyn CW (2003) Acquisition of polarized-light orientation in salmonids under laboratory conditions. Anim Behav 65:893–904. doi:10.1006/anbe.2003.2136
Pearce JM, Ward-Robinson J, Good M, Fussell C, Aydin A (2001) Influence of a beacon on spatial learning based on the shape of the test environment. J Exp Psychol 27:329–344
Redhead ES, Roberts A, Good M, Pearce JM (1997) Interaction between piloting and beacon homing by rats in a swimming pool. J Exp Psychol 23:340–350
Rossier J, Haeberli C, Schenk F (2000) Auditory cues support place navigation in rats when associated with a visual cue. Behav Brain Res 117:209–214
Rozhok A (2008) Orientation and navigation in vertebrates. Springer, Berlin, Heidelberg
Sabbah S, Lerner A, Erlick C, Shashar N (2005) Under water polarization vision—a physical examination. In: Pandalai SG (ed) Recent research developments in experimental and theoretical biology. TRN Press, Kerala, pp 123–176
Shashar N, Rutledge PS, Cronin TW (1996) Polarization vision in cuttlefish—a concealed communication channel? J Exp Biol 199:2077–2084
Shashar N, Hanlon RT, deM Petz A (1998) Polarization vision helps detect transparent prey. Nature 393:222–223
Shashar N, Hagan R, Boal JG, Hanlon RT (2000) Cuttlefish use polarization sensitivity in predation on silvery fish. Vis Res 40:71–75. doi:10.1016/S0042-6989(99)00158-3
Shashar N, Johnsen S, Lerner A, Sabbah S, Chiao CC, Mäthger LM, Hanlon RT (2011) Underwater linear polarization: physical limitations to biological functions. Phil Trans R Soc B 366:649–654. doi:10.1098/rstb.2010.0190
Shettleworth SJ (2010) Getting around: spatial cognition. In: Shettleworth SJ (ed) Cognition, evolution, and behavior, 2nd edn. Oxford University Press, New York, pp 261–312
Steck K, Hansson BS, Knaden M (2011) Desert ants benefit from combining visual and olfactory landmarks. J Exp Biol 214:1307–1312. doi:10.1242/jeb.053579
Wehner R, Michel B, Antonsen P (1996) Visual navigation in insects: coupling of egocentric and geocentric information. J Exp Biol 199:129–140
Acknowledgments
We thank the staff of the CREC for their technical assistance. This research was supported by a grant from the Ministère de l’Enseignement Supérieur et de la Recherche to L.C.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cartron, L., Darmaillacq, AS., Jozet-Alves, C. et al. Cuttlefish rely on both polarized light and landmarks for orientation. Anim Cogn 15, 591–596 (2012). https://doi.org/10.1007/s10071-012-0487-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-012-0487-9