Abstract
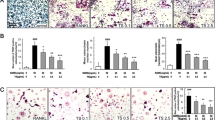
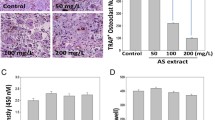
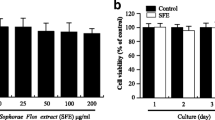
Since the 17th century Spica Prunella has been used as a medicinal herb. Dried and pulverized Spica Prunella samples were extracted and used in these experiments. In this study, the effects of Spica Prunella extract (SPE) on RANKL (receptor activator of nuclear factor κB ligand)-induced osteoclastogenesis were examined. Actin ring formation, a typical marker of osteoclastogenesis, was inhibited by SPE without any toxicity. There was also a marked inhibition in the formation of tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells in bone marrow-derived monocytes (BMMs). SPE also suppressed phosphorylation of c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinases (ERK) both of which are signals of the mitogen-activated protein kinases (MAPKs) signaling pathway. Additionally, SPE inhibited IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha) signaling pathway, which is an important factor in osteoclastogenesis. These results indicate that SPE might suppress osteoclast differentiation by inhibiting the phosphorylation of JNK and ERK in MAPK and NF-κB signaling pathways which act as messengers in the RANKL-induced osteoclast differentiation pathway. This means that SPE could potentially have great therapeutic usage in treating bone erosive diseases such as rheumatoid arthritis or in preventing metastasis associated with bone loss.
Similar content being viewed by others
References
Stains JP, Civitelli R. Cell-to-cell interactions in bone. Biochem. Bioph. Res. Co. 18: 721–727 (2005)
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 15: 337–342 (2003)
Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4: 638–649 (2003)
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 17: 165–176 (1998)
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20: 345–357 (1999)
Karlström E, Ek-Rylander B, Wendel M, Andersson G. RANKL induces components of the extrinsic coagulation pathway in osteoclasts. Biochem. Bioph. Res. Co. 394: 593–599 (2010)
Teitelbaum SL. Bone resorption by osteoclasts. Science 289: 1504–1508 (2000)
Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T. Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. P. Natl. Acad. Sci. USA 87: 7260–7264 (1990)
Nakao A, Fukushima H, Kajiya H, Ozeki S, Okabe K. RANKLstimulated TNF alpha production in osteoclast precursor cells promotes osteoclastogenesis by modulating RANK signaling pathways. Biochem. Bioph. Res. Co. 357: 945–950 (2007)
Lee SE, Chung WJ, Kwak HB, Chung CH, Kwack KB, Lee ZH, Kim HH. Tumor necrosis factoralpha supports the survival of osteoclasts through the activation of Akt and ERK. J. Biol. Chem. 276: 49343–49349 (2001)
Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem. Bioph. Res. Co. 305: 211–214 (2003)
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3: 889–901 (2002)
Armstrong AP, Tometsko ME, Glaccum M, Sutherland CL, Cosman D, Dougall WC. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J. Biol. Chem. 277: 44347–44356 (2002)
Lamothe B, Webster WK, Gopinathan A, Besse A, Campos AD, Darnay BG. TRAF6 ubiquitin ligase is essential for RANKL signaling and osteoclast differentiation. Biochem. Bioph. Res. Co. 359: 1044–1049 (2007)
Psotova J, Kolar M, Sousek J, Svagera Z, Vicar J, Ulrichova J. Biological activities of Prunella vulgaris extract. Phytother. Res. 17: 1082–1087 (2003)
Han EH, Choi JH, Hwang YP, Park HJ, Choi CY, Chung YC, Seo JK, Jeong HG. Immunostimulatory activity of aqueous extract isolated from Prunella vulgaris. Food. Chem. Toxicol. 47: 62–69 (2009)
Kim SY, Kim SH, Shin HY, Lim JP, Chae BS, Park JS, Hong SG, Kim MS, Jo DG, Park WH, Shin TY. Effects of Prunella vulgaris on mast cell-mediated allergic reaction and inflammatory cytokine production. Exp. Biol. Med. 232: 921–926 (2007)
Collins NH, Lessey EC, DuSell CD, McDonnell DP, Fowler L, Palomino WA, Illera MJ, Yu X, Mo B, Houwing AM, Lessey BA. Characterization of antiestrogenic activity of the Chinese herb, prunella vulgaris, using in vitro and in vivo (Mouse Xenograft) models. Biol. Reprod. 80: 375–383 (2009)
Park SJ, Kim DH, Lee IK, Jung WY, Park DH, Kim JM, Lee KR, Lee KT, Shin CY, Cheong JH, Ko KH, Ryu JH. The ameliorating effect of the extract of the flower of Prunella vulgaris var. lilacina on drug-induced memory impairments in mice. Food Chem. Toxicol. 48: 1671–1676 (2010)
Choi JH, Han EH, Hwang YP, Choi JM, Choi CY, Chung YC, Seo JK, Jeong HG. Suppression of PMA-induced tumor cell invasion and metastasis by aqueous extract isolated from Prunella vulgaris via the inhibition of NF-κB-dependent MMP-9 expression. Food. Chem. Toxicol. 48: 564–571 (2010)
Kim SW, Lee CH, Jung HK, Jo SS, Lee HK, Park YS. Effects of oleanolic acid and its derivatives on the differentiation of MC3T3-E1 osteoblastic cell. Korean J. Medicinal Crop. Sci. 19: 491–500 (2011)
Xu HX, Lee SH, Lee SF, White RL, Blay J. Isolation and characterization of an anti-HSV polysaccharide from Prunella vulgaris. Antiviral Res. 44: 43–54 (1999)
Ryu SY, Oak MH, Yoon SK, Cho DI, Yoo GS, Kim TS, Kim KM. Anti-allergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med. 66: 358–360 (2000)
Lamaison JL, Petitjean-Freytet C, Carnat A. Medicinal Lamiaceae with antioxidant properties, a potential source of rosmarinic acid. Pharm. Acta Helv. 66: 185–188 (1991)
Lee SE, Woo KM, Kim SY, Kim HM, Kwack K, Lee ZH, Kim HH. The phosphatidylinositol 3-kinase, p38, and extracellular signalregulated kinase pathways are involved in osteoclast differentiation. Bone 30: 71–77 (2002)
Kim K, Kim JH, Lee J, Jin HM, Lee SH, Fisher DE, Kook H, Kim KK, Choi Y, Kim N. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem. 280: 35209–35216 (2005)
Ha H, Ho J, Shin S, Kim H, Koo S, Kim IH, Kim C. Effects of Eucommiae Cortex on osteoblast-like cell proliferation and osteoclast inhibition. Arch. Pharm. Res. 391: 789–794 (2010)
Masuda K, Ikeuchi M, Koyama T, Yamaguchi K, Woo JT, Nishimura T, Yazawa K. Suppressive effects of Anoectochilus formosanus extract on osteoclast formation in vitro and bone resorption in vivo. J. Bone Miner. Metab. 26: 123–129 (2008)
Kim TH, Kim HJ, Lee SH, Kim SY. Potent inhibitory effect of Foeniculum vulgare Miller extract on osteoclast differentiation and ovariectomy-induced bone loss. Int. J. Mol. Med. 29: 1053–1059 (2012)
Nakashima T, Takayanagi H. New regulation mechanisms of osteoclast differentiation. Ann. NY. Acad. Sci. 1240: E13–E18 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ko, WK., Cho, JY., Moon, HJ. et al. Spica Prunella extract inhibits phosphorylation of JNK, ERK and IκBα signals during osteoclastogenesis. Food Sci Biotechnol 22, 1691–1698 (2013). https://doi.org/10.1007/s10068-013-0268-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-013-0268-5