Abstract
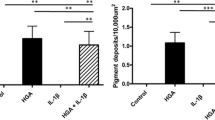
Alkaptonuria (AKU) is a rare genetic disease resulting in severe, rapidly progressing, early onset multi-joint osteoarthropathy. A potential therapy, nitisinone, is being trialled that reduces the causative agent; homogentisic acid (HGA) and in a murine model has shown to prevent ochronosis. Little is currently known about the effect nitisinone has on osteoarticular cells; these cells suffer most from the presence of HGA and its polymeric derivatives. This led us to investigate nitisinone’s effect on chondrocytes and osteoblast-like cells in an in vitro model. Human C20/A4 immortalized chondrocytes, and osteosarcoma cells MG63 cultured in DMEM, as previously described. Confluent cells were then plated into 24-well plates at 4 × 104 cells per well in varying concentrations of nitisinone. Cells were cultured for 7 days with medium changes every third day. Trypan blue assay was used to determine viability and the effect of nitisinone concentration on cells. Statistical analysis was performed using analysis of variance, and differences between groups were determined by Newman-Keuls post-test. Analysis of C20/A4 chondrocyte and MG63 osteoblast-like cell viability when cultured in different concentrations of nitisinone demonstrates that there is no statistically significant difference in cell viability compared to control cultures. There is currently no literature surrounding the use of nitisinone in human in vitro models, or its effect on chondrocytes or osteoblast like cells. Our results show that nitisinone does not appear detrimental to cell viability of chondrocytes or osteoblast-like cells, which adds to the evidence that this therapy could be useful in treating AKU.


Similar content being viewed by others
References
Mistry JB, Bukhari M, Taylor AM (2013) Alkaptonuria. Rare Dis 1, e27475. doi:10.4161/rdis.27475
Taylor AM, Boyde A, Wilson PJM, Jarvis JC, Davidson JS, Hunt JA et al (2011) The role of calcified cartilage and subchondral bone in the initiation and progression of ochronotic arthropathy in alkaptonuria. Arthritis Rheum 63(12):3887–3896
Grompe M, Lindstedt S, al-Dhalimy M, Kennaway NG, Papaconstantinou J, Torres-Ramos CA et al (1995) Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinemia type I. Nat Genet 10(4):453–460
Moran GR (2005) 4-Hydroxyphenylpyruvate dioxygenase. Arch Biochem Biophys 433(1):117–128
Introne WJ, Perry MB, Troendle J, Tsilou E, Kayser MA, Suwannarat P et al (2011) A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab 103(4):307–314
Preston AJ, Keenan CM, Sutherland H, Wilson PJ, Wlodarski B, Taylor AM et al (2014) Ochronotic osteoarthropathy in a mouse model of alkaptonuria, and its inhibition by nitisinone. Ann Rheum Dis 73(1):284–289
Ranganath LR, Milan AM, Hughes AT, Dutton JJ, Fitzgerald R, Briggs MC et al (2014) Suitability of nitisinone in alkaptonuria 1 (SONIA 1): an international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose–response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann Rheum Dis. doi:10.1136/annrheumdis-2014-206033, pii: annrheumdis-2014-206033
Tinti L, Taylor AM, Santucci A, Wlodarski B, Wilson PJ, Jarvis JC et al (2011) Development of an in vitro model to investigate joint ochronosis in alkaptonuria. Rheumatology (Oxford) 50(2):271–277
Gertsman I, Barshop BA, Panyard-Davis J, Gangoiti JA, Nyhan WL (2015) Metabolic effects of increasing doses of nitisinone in the treatment of alkaptonuria. JIMD Rep
Suzuki Y, Oda K, Yoshikawa Y, Maeda Y, Suzuki T (1999) A novel therapeutic trial of homogentisic aciduria in a murine model of alkaptonuria. J Hum Genet 44:79
Suwannarat P, O'Brien K, Perry MB, Lebring N, Bernardini I, Kaiser-Kupfer MI et al (2005) Use of nitisinone in patients with alkaptonuria. Metabolism 54:719
Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B (1992) Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340(8823):813–817
Lock EA, Ellis MK, Gaskin P, Robinson M, Auton TR, Provan WM et al (1998) From toxicological problem to therapeutic use: the discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J Inherit Metab Dis 21(5):498–506
Laschi M, Tinti L, Braconi D, Millucci L, Ghezzi L, Amato L et al (2012) Homogentisate 1,2 dioxygenase is expressed in human osteoarticular cells: implications in alkaptonuria. J Cell Physiol 227(9):3254–3257
Lock EA, Gaskin P, Ellis MK, Robinson M, Provan WM, Smith LL (1998) The effect of a low-protein diet and dietary supplementation of threonine on tyrosine and 2-(2-nitro-4-trifluoromethylbenzoyl) cyclohexane-1,3-dione-induced corneal lesions, the extent of tyrosinemia, and the activity of enzymes involved in tyrosine catabolism in the rat. Toxicol Appl Pharmacol 150(1):125–132
Gissen P, Preece MA, Willshaw HA, McKiernan PJ (2003) Ophthalmic follow-up of patients with tyrosinaemia type I on NTBC. J Inherit Metab Dis 26(1):13–16
El-Karaksy H, Rashed M, El-Sayed R, El-Raziky M, El-Koofy N, El-Hawary M et al (2010) Clinical practice. NTBC therapy for tyrosinemia type 1: how much is enough? Eur J Pediatr 169(6):689–693
Stewart RM, Briggs MC, Jarvis JC, Gallagher JA, Ranganath L (2014) Reversible keratopathy due to hypertyrosinaemia following intermittent low-dose nitisinone in alkaptonuria: a case report. JIMD Rep 17:1–6
Acknowledgments
The authors would like to thank the AKU Society, the Rosetrees Trust and University Hospitals Morecambe Bay NHS Foundation Trust for funding.
Disclosures
None.
Author contributions
AT and MB conceived the study and supervised JM and DJ.
AT, JM and DJ undertook experimental procedures.
All authors contributed to interpreting data, drafting, reading and approving the final version of the manuscript.
Role of the funding source
Funders had no input into study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mistry, J.B., Jackson, D.J., Bukhari, M. et al. Osteoarticular cells tolerate short-term exposure to nitisinone—implications in alkaptonuria. Clin Rheumatol 35, 513–516 (2016). https://doi.org/10.1007/s10067-015-2983-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-2983-1