Abstract
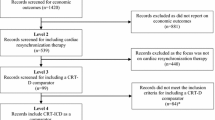
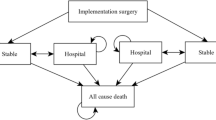
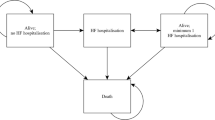
The aim of the present study was to perform a cost-effectiveness analysis (CEA) of ventricular assist devices (VAD) implantation surgery in the Japanese medical reimbursement system. The study group consisted of thirty-seven patients who had undergone VAD implantation surgery for dilated cardiomyopathy (n = 25; 67.6 %) or hypertrophic cardiomyopathy (n = 4; 10.8 %), and others (n = 8; 21.6 %). Quality-adjusted life years (QALYs) were calculated using the utility score and years of life. Medical reimbursement bills were chosen as cost indices. The observation period was the 12-month period after surgery. Then, the incremental cost-effectiveness ratio was calculated according to the VAD type. In addition, the prognosis after 36 months was estimated on the basis of the results obtained using the Markov chain model. The mean preoperative INTERMACS profile score was 2.35 ± 0.77. Our results showed that the utility score, which indicates the effectiveness of VAD implantation surgery, improved by 0.279 ± 0.188 (ΔQALY, p < 0.05). The cost of VAD implantation surgery was 313,282 ± 25,275 (ΔUS$/year) on the basis of medical reimbursement bills associated with therapeutic interventions. The calculated result of CEA was 364,501 ± 190,599 (ΔUS$/QALY). The improvement in the utility score was greater for implantable versus extracorporeal VADs (0.233 ± 0.534 vs. 0.371 ± 0.238) and ICER was 303,104 (ΔUS$/ΔQALY). Furthermore, when we estimated CEA for 36 months, the expected baseline value was 102,712 (US$/QALY). Therefore, VAD implantation surgery was cost effective considering the disease specificities.



Similar content being viewed by others
References
Saito S, Matsumiya G, Sakaguchi T, Miyagawa S, Yoshikawa Y, Yamauchi T, Kuratani T, Sawa Y. Risk factor analysis of long-term support with left ventricular assist system. Circ J. 2010;74:715–22.
Peura JL, Colvin-Adams M, Francis GS, Grady KL, Hoffman TM, Jessup M, John R, Kiernan MS, Mitchell JE, O’Connell JB, Pagani FD, Petty M, Ravichandran P, Rogers JG, Semigran MJ, Toole JM, American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012;126:2648–67.
Kyo S, Ono M, Nishimura T. The health care economics of heart transplants. 2009;44:10–7.
Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED, Shaw LJ. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–22.
Hutchinson J, Scott DA, Clegg AJ, Loveman E, Royle P, Bryant J, Colquitt JL. Cost-effectiveness of left ventricular-assist devices in end-stage heart failure. Expert Rev Cardiovasc Ther. 2008;6:175–85.
Sharples LD, Dyer M, Cafferty F, Demiris N, Freeman C, Banner NR, Large SR, Tsui S, Caine N, Buxton M. Cost-effectiveness of ventricularassist device use in the United Kingdom: results from the evaluation of ventricular assist device programme in the UK (EVAD-UK). J Heart Lung Transplant. 2006;25:1336–43.
Mahle WT, Ianucci G, Vincent RN, Kanter KR. Costs associated with ventricularassist device use in children. Ann Thorac Surg. 2008;86:1592–7.
Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, Freeman C. Evaluation of the ventricular assist device programme in the UK. Health Technol Assess. 2006;10:1–119, iii–iv.
Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, Bryant J. The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation. Health Technol Assess. 2005;9:1–132, iii–iv.
Kyo S, Nishimura T, Ono M. The social infrastructure of implantable ventricular assist devices. Special feature “Heading towards the era of implantable ventricular assist devices”. J Artif Organs. 2014;41:64–7.
Holman WL, Naftel DC, Eckert CE, Kormos RL, Goldstein DJ, Kirklin JK. Durability of left ventricular assist devices: interagency registry for mechanically assisted circulatory support (INTERMACS) 2006 to 2011. J Thorac Cardiovasc Surg. 2013;146:437–41.
Patlolla Vishnu, Patten Richard D, DeNofrio David, Konstam Marvin A, Krishnamani Rajan. The effect of ventricular assist devices on post-transplant mortality. J Am Coll Cardiol. 2009;53:264–71.
Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, Acker MA, Goldstein DL, Silvestry SC, Milano CA, Timothy Baldwin J, Pinney S, Eduardo Rame J, Miller MA. Interagency registry for mechanically assisted circulatory support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22.
Morshuis M, El-Banayosy A, Arusoglu L, Koerfer R, Hetzer R, Wieselthaler G, Pavie A, Nojiri C. European experience of DuraHeart magnetically levitated centrifugal left ventricular assist system. Eur J Cardiothorac Surg. 2009;35:1020–7.
Lahpor J, Khaghani A, Hetzer R, Pavie A, Friedrich I, Sander K, Strüber M. European results with a continuous-flow ventricular assist device for advanced heart-failure patients. Eur J Cardiothorac Surg. 2010;37:357–61.
Lalonde SD, Alba AC, Rigobon A, Ross HJ, Delgado DH, Billia F, McDonald M, Cusimano RJ, Yau TM, Rao V. Clinical differences between continuous flow ventricular assist devices: a comparison between HeartMate II and HeartWare HVAD. J Card Surg. 2013;28:604–10.
Kinoshita O, Naito N, Kondo H, et al. Interim outcomes of 20 cases of implantable left ventricular assist artificial hearts at The University of Tokyo Hospital. In: Symposium 03. 18th JACVAS Scientific Meeting; 2012.
Kubota K, Toda K, Yoshikawa Y, et al. The current state and challenges of waiting for a transplant at home after VAD implantation surgery at our hospital. In: Symposium 05. 18th JACVAS Scientific Meeting; 2012.
Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10.
Zahr F, Ootaki Y, Starling RC, et al. Preoperative risk factors for mortality after biventricular assist device implantation. J Card Fail. 2008;14:844–9.
Miller LW, Pagani FD, Russell SD, HeartMate II Clinical Investigators, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96.
Guidelines Manual-Appraising Orphan Drugs, NICE. 2006. http://www.nice.org.uk.
Takura Tomoyuki. Cost-effectiveness of hemodialysis in Japan. Contrib Nephrol. 2015;185:124–31.
Sutcliffe P, Connock M, Pulikottil-Jacob R, Kandala NB, Suri G, Gurung T, Grove A, Shyangdan D, Briscoe S, Maheswaran H, Clarke A. Clinical effectiveness and cost-effectiveness of second- and third-generation left ventricular assist devices as either bridge to transplant or alternative to transplant for adults eligible for heart transplantation: systematic review and cost-effectiveness model. Health Technol Assess. 2013;17(53):1–499, v–vi.
Acknowledgments
This study was structured on and funded by the Japanese Society for Medical Economics of Coronary Vascular Disease (JEC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tomoyuki Takura and Yoshiki Sawa received research funding from Terumo Co., Ltd. and Nipro Co., Ltd. Minoru Ono is advisory role of Sunmedical Co., Ltd. and he received research funding from Sunmedical Co., Ltd. The other authors declare that they have no conflict of interest.
Additional information
This article is from a study group of the Japanese Society for Medical Economics of Coronary Vascular Disease (JEC), Ventricular Assist Device Working Study Group.
Rights and permissions
About this article
Cite this article
Takura, T., Kyo, S., Ono, M. et al. Preliminary report on the cost effectiveness of ventricular assist devices. J Artif Organs 19, 37–43 (2016). https://doi.org/10.1007/s10047-015-0858-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-015-0858-5