Abstract
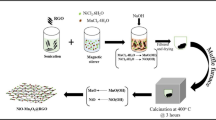
Hybrid material consisting of α-Fe2O3 and nitrogen-doped reduced graphene oxide (N-rGO/Fe2O3) for supercapacitor electrode material has been synthesized via an easy one-step hydrothermal method, where urea serves as nitrogen source, reducing agent and precipitant. As a result, the reduction and nitrogen doping of graphene oxide and the in situ formation of α-Fe2O3 are achieved simultaneously. The results show that the synthesized N-rGO/Fe2O3 composite exhibits much better electrochemical performance than the sample without nitrogen doping. Moreover, thanks to the positive synergetic effect between N-rGO and α-Fe2O3, the N-rGO/Fe2O3 composite shows superior electrochemical property, including high capacitance, excellent rate capability, and good cycle life. Consequently, the easy preparation approach in this work will be considered as an efficient pathway for the development of metal oxide or hydroxide/N-rGO electrode material for high-performance supercapacitors.

Nitrogen-doped reduced graphene oxide/α-Fe2O3 (N-rGO/Fe2O3) composite was prepared via easy one-step hydrothermal method, where urea served as nitrogen source, reducing agent, and precipitant. The presence of N-rGO was favored for the smaller size and more homogenous distribution of Fe2O3 nanoparticle. Due to the nitrogen doping and positive synergistic effect between N-rGO and Fe2O3, the obtained composite exhibited superior electrochemical performance.







Similar content being viewed by others
References
Yang SH, Song XF, Zhang P, Gao L (2013) ACS Appl Mater Inter 5:3317–3322
Yuan CZ, Li JY, Hou LR, Zhang XG, Shen LF, Lou XW (2012) Adv Funct Mater 22:4592–4597
Dai SG, Xi Y, Hu CG, Liu JL, Zhang KY, Yue XL, Cheng L (2013) J Mater Chem A 1:15530–15534
Liu JP, Jiang J, Cheng CW, Li HX, Zhang JX, Gong H, Fan HJ (2011) Adv Mater 23:2076–2081
Conway BE (1999) Electrochemical supercapacitors: scientific fundamentals and technological applications. Kluwer Academic/Plenum Publisher, New York
Zhang JL, Liu HD, Shi P, Li YJ, Huang LH, Mai WJ, Tan SZ, Cai X (2014) J Power Sources 267:356–365
Zhao X, Sanchez BM, Dobson PJ, Grant PS (2011) Nanoscale 3:839–855
Mai LQ, Yang F, Zhao YL, Xu X, Xu L, Luo YZ (2011) Nat Commun 2, doi: 10.1038/ncomms1387
Lu XH, Yu MH, Zhai T, Wang GM, Xie SL, Liu TY, Liang CL, Tong YX, Li Y (2013) Nano Lett 13:2628–2633
Reddy MV, Yu T, Sow CH, Shen ZX, Lim CT, Rao GVS, Chowdari BVR (2007) Adv Funct Mater 17:2792–2799
Wu NL, Wang SY, Han CY, Wu DS, Shiue LR (2003) J Power Sources 113:173–178
Nagarajan N, Zhitomirsky I (2006) J Appl Electrochem 36:1399–1405
Xie KY, Li J, Lai YQ, Lu W, Zhang ZA, Liu YX, Zhou LM, Huang HT (2011) Electrochem Commun 13:657–660
Zhao X, Johnston C, Grant PS (2009) J Mater Chem 19:8755–8760
Allen MJ, Tung VC, Kaner RB (2010) Chem Rev 110:132–145
Lee KK, Deng S, Fan HM, Mhaisalkar S, Tan HR, Tok ES, Loh KP, Chin WS, Sow CH (2012) Nanoscale 4:2958–2961
Jeong HM, Lee JW, Shin WH, Choi YJ, Shin HJ, Kang JK, Choi JW (2011) Nano Lett 11:2472–2477
Qiu YC, Zhang XF, Yang SH (2011) Phys Chem Chem Phys 13:12554–12558
Lin ZY, Liu Y, Yao YG, Hildreth OJ, Li Z, Moon K, Wong CP (2011) J Phys Chem C 115:7120–7125
Wu ZS, Ren W, Xu L, Li F, Cheng HM (2011) ACS Nano 5:5463–5471
Lv RT, Cui TX, Jun MS, Zhang Q, Cao AY, Su DS, Zhang ZJ, Yoon SH, Miyawaki J, Mochida I, Kang FY (2011) Adv Funct Mater 21:999–1006
Hummers WS, Offeman RE (1958) J Am Chem Soc 80:1339
Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Chizhik SA, Buzaneva EV, Gorchinskly AD (1999) Chem Mater 11:771–778
Sun L, Wang L, Tian CG, Tan TX, Xie Y, Shi KY, Li MT, Fu HG (2012) RSC Adv 2:4498–4506
Shin WH, Jeong HM, Kim BG, Kang JK, Choi JW (2012) Nano Lett 12:2283–2288
Deng DH, Pan XL, Yu LA, Cui Y, Jiang YP, Qi J, Li WX, Fu Q, Ma XC, Xue QK, Sun GQ, Bao XH (2011) Chem Mater 23:1188–1193
Lambert TN, Chavez CA (2009) J Phys Chem C 113:19812–19823
Jeong HK, Lee YP, Lahaye RJWE, Park MH, An KH, Kim IJ, Yang CW, Park CY, Ruoff RS, Lee YH (2008) J Am Chem Soc 130:1362–1366
Guo HL, Wang XF, Qian QY, Wang FB, Xia XH (2009) ACS Nano 3:2653–2659
Xu YY, Yang S, Zhang GY, Sun YQ, Gao DZ, Sun YX (2011) Mater Lett 65:1911–1914
Xue YH, Liu J, Chen H, Wang RG, Li DQ, Qu J, Dai LM (2012) Angew Chem Int Ed 51:12124–12127
Hassan MF, Guo Z, Chen Z, Liu H (2011) Mater Res Bull 46:858–864
Jin Z, Yao J, Kittrell C, Tour JM (2011) ACS Nano 5:4112–4117
Wang Y, Shao YY, Matson DW, Li JH, Lin YH (2010) ACS Nano 4:1790–1798
Malitesta C, Losito I, Sabbatini L, Zambonin PG (1995) J Electron Spectrosc Relat Phenom 76:629–634
Casanovas J, Ricart JM, Rubio J, Illas F, Jimenez-Mateos JM (1996) J Am Chem Soc 118:8071–8076
Hulicova-Jurcakova D, Seredych M, Lu GQ, Bandosz TJ (2009) Adv Funct Mater 19:438–447
Lota G, Lota K, Frackowiak E (2007) Electrochem Commun 9:1828–1832
Zhu XJ, Zhu W, Murali YS, Stollers MD, Ruoff RS (2011) ACS Nano 5:3333–3338
Wu CZ, Yin P, Zhu X, Ouyang C, Xie Y (2006) J Phys Chem B 110:17806–17812
Wang DW, Li YQ, Wang QH, Wang TM (2012) J Solid State Electrochem 16:2095–2102
Low QX, Ho GW (2014) Nano Energy 5:28–35
Zhu W, Murali S, Stollers MD, Ganesh KJ, Cai WW, Ferreira PJ, Ruoff RS (2011) Science 332:1537–1541
Chen S, Zhu JW, Wu XD, Han QF, Wang X (2010) ACS Nano 4:2822–2830
Wang Z, Ma CY, Wang HL, Liu ZH, Hao ZP (2013) J Alloy Compd 552:486–491
Yang WL, Gao Z, Wang B, Liu LH (2013) Solid State Sci 20:46–53
Wang DW, Wang QH, Wang TM (2011) Nanotechnology 22:135604
Xu L, Xia JX, Xu H, Yin S, Wang K, Huang LY, Wang LG, Li HM (2014) J Power Sources 245:866–874
Pandey GP, Hashmi SA, Kumar Y (2010) J Electrochem Soc 157:A105–A114
Fang DL, Chen ZD, Liu X, Wu ZF, Zheng CH (2012) Electrochim Acta 81:321–329
Acknowledgements
The authors would like to thank the National Nature Science Foundation of China (Nos. 20801023, 21271087, and 51172099), the Fundamental Research Funds for the Central Universities (Nos. 51208024 and 11612109), and the Research and innovation project of Jinan University for Excellent Master (201415).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H.D., Zhang, J.L., Xu, D.D. et al. Easy one-step hydrothermal synthesis of nitrogen-doped reduced graphene oxide/iron oxide hybrid as efficient supercapacitor material. J Solid State Electrochem 19, 135–144 (2015). https://doi.org/10.1007/s10008-014-2580-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-014-2580-2