Abstract
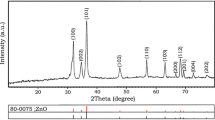
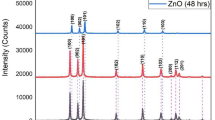
Two zinc oxide (ZnO) nanoparticles ZS with hexagonal sheet and ZN having nanorod-like morphologies were prepared by precipitation technique using two different hydrolyzing agents viz. water (ZS) and ethanol (ZN). The physicochemical properties of both of the as-synthesized ZnO nanoparticles were investigated by UV–vis, powder X-ray diffraction (pXRD) and field emission scanning microscopy (FESEM). The as-synthesized ZnO nanoparticulates were successfully employed as photoanode material in dye-sensitized solar cells (DSSCs). The photoanode fabricated by using ZN in DSSC showed two times better cell efficiency with enhanced photocurrent and open circuit potential in comparison to the photoanode fabricated using ZS. The improved efficiency of ZN over ZS is due to high active surface area for greater dye loading.

The enhanced efficiency has been obtained by employing ZnO nanostructures (nanosheets and short rods) as photoanode in dye-sensitized solar cells.









Similar content being viewed by others
References
O’Regan B, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Zhang Q, Cao G (2011) Nanostructured photoelectrodes for dye-sensitized solar cells. Nano Today 6:91–109
Duong TT, Choi HJ, He QJ, Le AT, Yoon SG (2013) Enhancing the efficiency of dye sensitized solar cells with an SnO2 blocking layer grown by nanocluster deposition. J Alloys Compd 561:206–210
Xu F, Zhang X, Wu Y, Wu D, Gao Z, Jiang K (2013) Facile synthesis of TiO2 hierarchical microspheres assembled by ultrathin nanosheets for dye-sensitized solar cells. J Alloys Compd 574:227–232
Hagfeldt A, Grätzel M (1995) Light-induced redox reactions in nanocrystalline systems. Chem Rev 95:49–68
Kalyansundaramand K, Grätzel M (1998) Applications of functionalized transition metal complex in photonic and optoelectronic devices. Coord Chem Rev 177:347–414
Kong FT, Dai SY, Wang KJ (2007) Review of recent progress in dye-sensitized solar cells. Adv OptoElectron 1–13
Gratzel M (2003) Dye-sensitized solar cells. J Photochem Photobiol C Photochem Rev 4:145–153
Polo AS, Itokazu MK, Iha NYM (2004) Metal complex sensitizers in dye-sensitized solar cells. Coord Chem Rev 248:1343–1361
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Zhang Q, Dandeneau CS, Zhou X, Cao G (2009) ZnO nanostructures for dye-sensitized solar cells. Adv Mater 21:4087–4108
Xu F, Sun L (2011) Solution-derived ZnO nanostructures for photoanodes of dye-sensitized solar cells. Energy Environ Sci 4:818–841
Hu X, Heng B, Chen X, Wang B, Sun D, Sun Y, Zhou W, Tang Y (2012) Ultralong porous ZnO nanobelt arrays grown directly on fluorine-doped SnO2 substrate for dye-sensitized solar cells. J Power Sources 217:120–127
Sakai N, Miyasaka T, Murakami TN (2013) Efficiency enhancement of ZnO-based dye-sensitized solar cells by low-temperature TiCl4 treatment and dye optimization. J Phys Chem C 117:10949–10956
Meng XQ, Zhao DX, Zhang JY, Shan DZ, Lu YM, Liu YC, Fan XW (2005) Growth temperature controlled shape variety of ZnO nanowires. Chem Phys Lett 407:91–94
Umar A, Lee S, Lee YS, Nahm KS, Hahn YB (2005) Star-shaped ZnO nanostructures on silicon by cyclic feeding chemical vapor deposition. J Cryst Growth 277:479–484
Pan ZW, Dai ZR, Wang ZL (2001) Nanobelts of semiconducting oxides. Science 291:1947–1949
Wahab R, Ansari SG, Kim YS, Seo HK, Shin HS (2007) Room temperature synthesis of needle-shaped ZnO nanorods via sonochemical method. Appl Surf Sci 253:7622–7626
Wahab R, Ansari SG, Kim YS, Seo HK, Kim GS, Khang G, Shin HS (2007) Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater Res Bull 42:1640–1648
Wahab R, Ansari SG, Kim YS, Khang G, Shin HS (2008) Effect of hydroxylamine hydrochloride on the floral decoration of zinc oxide synthesized by solution method. Appl Surf Sci 254:2037–2042
Meulenkamp EA (1998) Synthesis and growth of ZnO nanoparticles. J Phys Chem B 102:5566–5572
Spanhel L, Anderson MA (1991) Semiconductor clusters in the sol-gel process: quantized aggregation, gelation, and crystal growth in concentrated zinc oxide colloids. J Am Chem Soc 113:2826–2833
Zhang J, Sun L, Yin J, Su H, Liao CS, Yan C (2002) Control of ZnO morphology via a simple solution route. Chem Mater 14:4172–4177
Nyffenegger RM, Craft B, Shaaban M, Gorer S, Erley G, Penner RM (1998) A hybrid electrochemical/chemical synthesis of zinc oxide nanoparticles and optically intrinsic thin films. Chem Mater 10:1120–1129
Brinker CJ, Scherer GW (1990) Sol–gel science: the physics and chemistry of sol–gel processing. Academic, San Diego
Xu L, Hu YL, Pelligra C et al (2009) ZnO with different morphologies synthesized by solvothermal methods for enhanced photocatalytic activity. Chem Mater 21:2875–2885
Khoza PB, Moloto MJ, Sikhwivhilu LM (2011) The effect of solvents, acetone, water, and ethanol, on the morphological and optical properties of ZnO nanoparticles prepared by microwave. J Nanotechnol 2012:1–6
Koch U, Fojtik A, Weller H, Henglein A (1985) Photochemistry of semiconductor colloids, preparation of extremely small ZnO particles, fluorescence phenomena and size quantization effects. Chem Phys Lett 122:507–510
Wang Q, Moser JE, Gratzel M (2005) Electrochemical impedance spectroscopic analysis of dye-sensitized solar cells. J Phys Chem B 109:14945–14953
Lee KM, Hu CW, Chen HW, Ho KC (2008) Incorporating carbon nanotubes in a low-temperature fabrication process for dye-sensitized TiO2 solar cells. Sol Energy Mater Sol Cells 92:1628–1633
Yanagida S, Yu YH, Manseki K (2009) Iodine/iodide-free dye-sensitized solar cells. Acc Chem Res 42:1827–1838
Han J, Fan F, Xu C, Lin S, Wei M, Duan X, Wang ZL (2010) ZnO nanotube-based dye-sensitized solar cell and its application in self-powered devices. Nanotechnology 21:405203–405207
Bahadur L, Kushwaha S (2012) Highly efficient nanocrystalline ZnO thin films prepared by a novel method and their application in dye-sensitized solar cells. Appl Phys A 109:655–663
Kumar RS, Sudhagar P, Matheswaran P, Sathyamoorthy R, Kang YS (2010) Influence of seed layer treatment on ZnO growth morphology and their device performance in dye-sensitized solar cells. Mater Sci Eng B 172:283–288
Umar A (2009) Growth of comb-like ZnO nanostructures for dye sensitized solar cells applications. Nanoscale Res Lett 4:1004–1008
Cadena GJ, Comini E, Ferroni M, Vomiero A, Sberveglieri G (2010) Synthesis of different ZnO nanostructures by modified PVD process and potential use for dye-sensitized solar cells. Mater Chem Phys 124:694–698
Baxter JB, Aydil SE (2006) Dye-sensitized solar cells based on semiconductor morphologies with ZnO nanowires. Sol Energy Mater Sol Cells 90:607–622
Acknowledgments
Authors are grateful to the Department of Science and Technology and INSA, New Delhi for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• The high quality ZnO nanostructures synthesized using water (ZS) and ethanol (ZN) as two hydrolyzing agents of different polarity.
• The efficiency for the cell using ZN as photoanode shows two time better output in comparison to ZS.
• IPCE = 41 % has been obtained with the use of ZN as photoanode.
Rights and permissions
About this article
Cite this article
Chauhan, R., Kumar, A., Umarji, G.G. et al. Comparison of optical and photovoltaic properties of ZnO chemically synthesized by using different hydrolyzing agents. J Solid State Electrochem 19, 161–168 (2015). https://doi.org/10.1007/s10008-014-2568-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-014-2568-y