Abstract
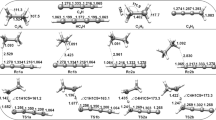
We present a theoretical study on the detailed mechanism and kinetics of the H+HCN →H+HNC process. The potential energy surface was calculated at the complete basis set quantum chemical method, CBS-QB3. The vibrational frequencies and geometries for four isomers (H2CN, cis-HCNH, trans-HCNH, CNH2), and seven saddle points (TSn where n = 1 − 7) are very important and must be considered during the process of formation of the HNC in the reaction were calculated at the B3LYP/6-311G(2d,d,p) level, within CBS-QB3 method. Three different pathways (PW1, PW2, and PW3) were analyzed and the results from the potential energy surface calculations were used to solve the master equation. The results were employed to calculate the thermal rate constant and pathways branching ratio of the title reaction over the temperature range of 300 up to 3000 K. The rate constants for reaction H + HCN → H + HNC were fitted by the modified Arrhenius expressions. Our calculations indicate that the formation of the HNC preferentially occurs via formation of cis–HCNH, the fitted expression is k P W2(T) = 9.98 × 10−22 T 2.41 exp(−7.62 kcal.mol−1/R T) while the predicted overall rate constant k O v e r a l l (T) = 9.45 × 10−21 T 2.15 exp(−8.56 kcal.mol−1/R T) in cm 3 molecule −1 s −1.

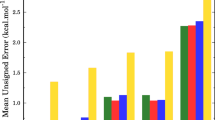
(a) Potential energy surface, (b) thermal rate constants as a function of temperature and (c) the branching ratios (%) of PW1, PW2, PW3 pathways involved in rm H + HCN → H + HNC process.






Similar content being viewed by others
References
Haynes BS (1977) Combust Flame 28:113
West GA, Berry MJ (1974) J Chem Phys 61:4700
Strobel DF (1983) Int Rev Phys Chem 3:145
Sumathi R, Nguyen MT (1998) J Phys Chem A 102:8013
Herbst E (1978) Ap J 222:508
Hebrard E, Dobrijevic M, Loison JC, Bergeat A, Hickson KM (2012) Astron Astrophys 541:13
Petrie S (2002) J Phys Chem A 106(1):1181
ter Horst MA, Schatz GC, Harding LB (1996) J Chem Phys 105:558
Goldsmith PF, Langer WD, Ellder J, Irvine W, Kollberg E (1981) Astrophys J 249:521
Wootten A, Evans NJ, Snell RI, Vanden PB (1978) Ap J Lett 225:L143
Glowacki DR, Liang CH, Morley C, Pilling MJ, Robertson SH (2012) J Phys Chem A 116:9545
Wood GPF, Radom L, Petersson GA, Barnes EC, Frisch MJ, Montgomery JA Jr (2006) J Chem Phys 125(094106):1–16
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2009) Gaussian 09, Gaussian, Inc., Wallingford
Bartis JT, Widom B (1974) J Chem Phys 60:3474
Baulch DL, Cobos CJ, Cox RA, Frank P, Hayman G, Just T, Kerr JA, Murrells T, Troe MJPJ, Walker RW, Warnatz J (1994) J Phys Chem Ref Data 23:847
Wang JH, Liu K, Schatz GC, ter Horst M (1997) J Chem Phys 107:7869
Zhu W, Zhang JZH, Zhang YC, Zhang YB, Zhan LX, Zhang SL, Zhang DH (1998) J Chem Phys 108:3509
Jiang B, Guo H (2013) J Chem Phys 139:224310
Wang X, Bowman M (2013) J Chem Theory Comput 9:901
Bair RA, Dunning TH (1985) J Chem Phys 82:2280
Talbi D, Ellinger Y (1996) Chem. Phys. Lett 263:385
Mills P, Jentz D, Trenary M (1997) J Am Chem Soc 119:9002
Hu X, Yin J, Meyer RJ, Trenary M (2015) J Phys Chem C 119:14506
Nakagawa T, Morino Y (1969) Bull Chem Soc Jpn 42:2212
Acknowledgments
This work was supported by a MCTI-PCI grant, Institutional Process Number 454779/2015-1, Individual Process Number 170134/2016-4.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: During the steps of corrections, the title went wrong. The correct title is shown above.
This paper belongs to Topical Collection VI Symposium on Electronic Structure and Molecular Dynamics – VI SeedMol
An erratum to this article is available at http://dx.doi.org/10.1007/s00894-017-3369-x.
Rights and permissions
About this article
Cite this article
Correa, E., da Silva, W.B., P. Barreto, P.R. et al. Theoretical study of the H + HCN → H + HNC process. J Mol Model 23, 169 (2017). https://doi.org/10.1007/s00894-017-3335-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3335-7