Abstract
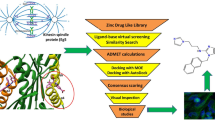
In this work, through a docking analysis of compounds from the ZINC chemical library on human β-tubulin using high performance computer cluster, we report new polycyclic aromatic compounds that bind with high energy on the colchicine binding site of β-tubulin, suggesting three new key amino acids. However, molecular dynamic analysis showed low stability in the interaction between ligand and receptor. Results were confirmed experimentally in in vitro and in vivo models that suggest that molecular dynamics simulation is the best option to find new potential β-tubulin inhibitors.

Bennett’s acceptance ratio (BAR) method






Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Abolhasani H, Zarghi A, Hamzeh-Mivehroud M, Alizadeh AA, Shahbazi Mojarrad J, Dastmalchi S (2015) In-silico investigation of tubulin binding modes of a series of novel antiproliferative spiroisoxazoline compounds using docking studies. Iran J Pharm Res 14(1):141–147
Ayaz P, Ye X, Huddleston P, Brautigam CA, Rice LM (2012) A TOG: αβ-tubulin complex structure reveals conformation-based mechanisms for a microtubule polymerase. Science 337(6096):857–860
Lippert JW 3rd (2007) Vascular disrupting agents. Bioorg Med Chem 15(2):605–615
Abro A, Kulsoom S, Riaz N (2013) Pharmacophore model generation for microtubule-stabilizing anti-mitotic agents (MSAAs) against ovarian cancer. Med Chem Res 22(9):4322–4330
Kars MD, Işeri ÖD, Gündüz U (2011) A microarray based expression profiling of paclitaxel and vincristine resistant MCF-7 cells. Eur J Pharmacol 657(1):4–9
Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M (2004) Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428(6979):198–202
Greene LM, Nathwani SM, Bright SA, Fayne D, Croke A, Gagliardi M, Zisterer DM (2010) The vascular targeting agent combretastatin-A4 and a novel cis-restricted β-lactam analogue, CA-432, induce apoptosis in human chronic myeloid leukemia cells and ex vivo patient samples including those displaying multidrug resistance. J Pharmacol Exp Ther 335(2):302–313
Greene LM, Carr M, Keeley NO, Lawler M, Meegan MJ, Zisterer DM (2011) BubR1 is required for the mitotic block induced by combretastatin-A4 and a novel cis-restricted ß-lactam analogue in human cancer cells. Int J Mol Med 27(5):715–723
O’Boyle NM, Carr M, Greene LM, Bergin O, Nathwani SM, McCabe T, Meegan MJ (2010) Synthesis and evaluation of azetidinone analogues of combretastatin A-4 as tubulin targeting agents. J Med Chem 53(24):8569–8584
O’Boyle NM, Knox AJ, Price TT, Williams DC, Zisterer DM, Lloyd DG, Meegan MJ (2011) Lead identification of β-lactam and related imine inhibitors of the molecular chaperone heat shock protein 90. Bioorg Med Chem 19(20):6055–6068
Loong HH, Yeo W (2014) Microtubule-targeting agents in oncology and therapeutic potential in hepatocellular carcinoma. OncoTargets Ther 7:575–585
Kumar A, Zhang KY (2015) Hierarchical virtual screening approaches in small molecule drug discovery. Methods 71:26–37
Segura-Cabrera A, Rodríguez-Pérez MA (2008) Structure-based prediction of Mycobacterium tuberculosis shikimate kinase inhibitors by high-throughput virtual screening. Bioorg Med Chem Lett 18(11):3152–3157
Kumar V, Krishna S, Siddiqi MI (2015) Virtual screening strategies: recent advances in the identification and design of anti-cancer agents. Methods 71:64–70
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Mahaddalkar T, Suri C, Naik PK, Lopus M (2015) Biochemical characterization and molecular dynamic simulation of β-sitosterol as a tubulin-binding anticancer agent. Eur J Pharmacol 760:154–162
Vilar S, Cozza G, Moro S (2008) Medicinal chemistry and the molecular operating environment (MOE): application of QSAR and molecular docking to drug discovery. Curr Top Med Chem 8(18):1555–1572
Gupta AK, Varshney K, Saxena AK (2012) Toward the identification of a reliable 3D QSAR pharmacophore model for the CCK2 receptor antagonism. J Chem Inf Model 52(5):1376–1390
Penthala NR, Zong H, Ketkar A, Madadi NR, Janganati V, Eoff RL, Crooks PA (2015) Synthesis, anticancer activity and molecular docking studies on a series of heterocyclic trans-cyanocombretastatin analogues as antitubulin agents. Eur J Med Chem 92:212–220
Kendrick I, Kumari D, Yakaboski A, Dimakis N, Smotkin ES (2010) Elucidating the ionomer-electrified metal interface. J Am Chem Soc 132(49):17611–17616
Chopra A, Anderson A, Giardina C (2014) Novel piperazine-based compounds inhibit microtubule dynamics and sensitize colon cancer cells to tumor necrosis factor-induced apoptosis. J Biol Chem 289(5):2978–2991
Webber M, Dimakis N, Kumari D, Fuccillo M, Smotkin ES (2010) Mechanically coupled internal coordinates of ionomer vibrational modes. Macromolecules 43(13):5500–5502
DeLano WL (2002) The PyMOL molecular graphics system. https://www.pymol.org/. Accessed: 5 April 2016
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4(1):17
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Guthikonda SM (2005) Kohonen Self-Organizing Maps. Wittenberg University. http://www.shy.am/wp-content/uploads/2009/01/kohonen-self-organizing-maps-shyam-guthikonda.pdf. Accessed: 5 April 2016
Kohonen T (1998) The self-organizing map. Neurocomputing 21(1):1–6
Chen K, Huzil JT, Freedman H, Ramachandran P, Antoniou A, Tuszynski JA, Kurgan L (2008) Identification of tubulin drug binding sites and prediction of relative differences in binding affinities to tubulin isotypes using digital signal processing. J Mol Graph Model 27(4):497–505
Webb B, Sali A (2002) Comparative Protein Structure Modeling Using MODELLER. In: Current Protocols in Bioinformatics. Wiley, New York. doi:10.1002/cpbi.3
National Center for Biotechnology Information. tubulin beta-2B chain [Homo sapiens] http://www.ncbi.nlm.nih.gov/protein/29788768/may 2015. Accessed: 5 March 2016
Irwin JJ, Shoichet BK (2005) ZINC-a free database of commercially available compounds for virtual screening. J Chem Inf Model 45(1):177–182
Bañuelos-Hernández AE, Mendoza-Espinoza JA, Pereda-Miranda R, Cerda-García-Rojas CM (2014) Studies of (−)-Pironetin Binding to α-Tubulin: conformation, docking, and molecular dynamics. J Org Chem 79(9):3752–3764
Patel B, Jajoo A, Tibrewal Y, Joshi A (2015) An Efficient Parallel Algorithm for Self-Organizing Maps using MPI-OpenMP based Cluster. In: IJCA Proceedings on International Conference on Advanced Computing and Communication Techniques for High Performance Applications ICACCTHPA 2014 (2):5–9
Bhattacharyya B, Panda D, Gupta S, Banerjee M (2008) Anti‐mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med Res Rev 28(1):155–183
Iwasa K, Kamigauchi M, Takao N, Cushman M, Wong WC, J-k C (1988) Formation of benzo [c] phenanthridines by oxidative C-N bond fission of protoberberines followed by intramolecular recyclization in cell cultures of corydalisincisa. Tetrahedron Lett 29(49):6457–6460
Khilya O, Volovnenko T, Volovenko YM (2006) The reaction of 2-hetarylacetonitriles with heterocyclic haloaldehydes. Chem Heterocycl Compd 42(10):1311–1324
Kulkarni AR, Thakur GA (2013) Microwave-assisted expeditious and efficient synthesis of cyclopentene ring-fused tetrahydroquinoline derivatives using three-component Povarov reaction. Tetrahedron Lett 54(48):6592–6595
Becerra D, Insuasty B, Cobo J, Glidewell C (2013) 3, 3′-[(1RS, 3SR)-2-Oxocyclohexane-1, 3-diyl] bis [(3RS, 3′ SR)-3-hydroxyindolin-2-one] dihydrate: organic layers of R22 (8), R22 (16) and R66 (40) rings linked by tetrameric water aggregates. Acta Cryst C 69(9):1081–1084
Yang SW, Ho GD, Tulshian D, Bercovici A, Tan Z, Hanisak J, Brumfield S, Matasi J, Sun X, Sakwa SA, Herr RJ, Zhou X, Bridal T, Urban M, Vivian J, Rindgen D, Sorota S (2014) Bioavailable pyrrolo-benzo-1,4-diazines as Na(v)1.7 sodium channel blockers for the treatment of pain. Bioorg Med Chem Lett 24(21):4958–4962
Olekhnovich E, Boroshko S, Korobka I, Metelitsa A, Olekhnovich L (2001) Acetals and vinyl ethers of unsaturated aldehydes and ketones in the new syntheses of heterocyclic compounds: XII. New alternatives of acid condensation of cyclohexane-1, 4-diones with hydroxyarylaldehydes under dehydration conditions. Fluorecence spectra of the products. Russ J Org Chem 37(4):527–538
Sander T (2001) OSIRIS property explorer. Actelion Pharmaceuticals, Allschwil, Switzerland
Dipple A, Khan QA, Page JE, Ponten I, Szeliga J (1999) DNA reactions, mutagenic action and stealth properties of polycyclic aromatic hydrocarbon carcinogens (review). Int J Oncol 14(1):103–112
Pineda O, Farras J, Maccari L, Manetti F, Botta M, Vilarrasa J (2004) Computational comparison of microtubule-stabilising agents laulimalide and peloruside with taxol and colchicine. Bioorg Med Chem Lett 14(19):4825–4829
Naik PK, Santoshi S, Rai A, Joshi HC (2011) Molecular modelling and competition binding study of Br-noscapine and colchicine provide insight into noscapinoid-tubulin binding site. J Mol Graph Model 29(7):947–955
O’Boyle NM, Pollock JK, Carr M, Knox AJ, Nathwani SM, Wang S, Caboni L, Zisterer DM, Meegan MJ (2014) beta-Lactam estrogen receptor antagonists and a dual-targeting estrogen receptor/tubulin ligand. J Med Chem 57(22):9370–9382
Acknowledgments
F.E.O.S. is the recipient of a scholarship (No. 419250) from Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico. We wish to express our gratitude to the Universidad Autonoma de Nuevo Leon (UANL), Instituto Politecnico Nacional (IPN), University of Texas Rio Grande Valley (UTRGV) and the University of Konstanz (UK) for their technical and financial support. In particular to Thomas U. Mayer for his support in the in vitro and in vivo assays, and Robert C. Jackson from UTRGV. G.R. holds a scholarship from the “Comision de Operacion y Fomento de Actividades Academicas” (COFAA-IPN) and the “Programa de Estimulos al Desempeño de los Investigadores” (EDI-IPN). All participants declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olazarán, F.E., García-Pérez, C.A., Bandyopadhyay, D. et al. Theoretical and experimental study of polycyclic aromatic compounds as β-tubulin inhibitors. J Mol Model 23, 85 (2017). https://doi.org/10.1007/s00894-017-3256-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3256-5