Abstract
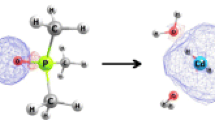
Calcium complexes with bidentate carbonyl ligands are important in biological systems, medicine and industry, where the concentration of Ca2+ is controlled using chelating ligands. The exchange of two water molecules of [Ca(H2O)6]2+ for one bidentate monosubstituted and homo disubstituted dicarbonyl ligand was investigated using the B3LYP/6-311++G(d,p) method. The ligand substituents NH2, OCH3, OH, CH3, H, F, Cl, CN and NO2 are functional groups with distinct electron-donating and -withdrawing effects that bond directly to the sp2 C atom of the carbonyl group. The geometry, charge and energy characteristics of the complexes were analyzed to help understand the effects of substituents, spacer length and chelation. Coordination strength was quantified in terms of the enthalpy and free energy of the exchange reaction. The most negative enthalpies were calculated for the coordination of bidentate ligands containing three to five methylene group spacers between carbonyls. The chelate effect contribution was analyzed based on the thermochemistry. The electronic character of the substituent modulates the strength of binding to the metal cation, as ligands containing electron-donor substituents coordinate stronger than those with electron-acceptor substituents. This is reflected in the geometric (bond length and chelating angle), electronic (atomic charges) and energetic (components of the total interacting energy) characteristics of the complexes. Energy decomposition analysis (EDA)—an approach for partitioning of the energy into its chemical origins—shows that the electrostatic component of the coordination is predominant, and yields relevant contribution of the covalent term, especially for the electron-withdrawing substituted ligands. The chelate effect of the bidentate ligands was noticeable when compared with substitution by two monodentate ligands.

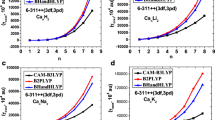
The affinity of 18 bidentate carbonyl ligands toward the [Ca(H2O)4]2+ cation is evaluated in terms of energetic, geometric and electronic parameters of the isolated ligands and the substituted aqua complexes. The electronic effects—inductive and mesomeric—intrinsic to the molecular structure of each ligand are found to modulate the strength of the metal-ligand interaction. The effects of polysubstitution, chelation and the length of the alkyl spacers between the anchor points of the ligand are also analyzed.





Similar content being viewed by others
References
Doskocz M, Kubas K, Frackowiak A, Gancarz R (2009) Polyhedron 28:2201–2205
Yi P, Liu Z, Wang Z, Zhou J, Hou B, Li Q (2013) Int J Quantum Chem 113:1316–1324
Meng L, Lin Z (2014) Comput Theor Chem 1039:1–10
Gerber S, Frohlich M, Lichtenberg-Fraté H, Shabala S, Shabala L, Klipp E (2016) PLoS Comput Biol 12:1
Li H, Han D, Pauletti GM, Steckl AJ (2014) Lab Chip 14:4025–4041
Fukata Y, Amano M, Kaibuchi K (2001) Trends Pharmacol Sci 22:32–39
Cape RA, Terrar DA (2015) Front Physiol 6:80
Zhou W, Tang GW, Altman RB (2015) J Chem Inf Model 55:1663–1672
Bristow SM, Gamble GD, Stewart A, Horne AM, Reid IR (2015) Br J Nutr 114:1868–1874
Suzuki T, Kajita Y, Katsuamta H, Suzuki K (2015) J Nutr Sci Vitaminol 61:382–390
Bonan RF, Bonan PRF, Batista AUD, Oliveira JE, Menezes RR, Medeiros ES (2014) Cerâmica 60:402–410
Riccardis F, Izzol I, Montesarchio D, Tecilla AP (2013) Acc Chem Res 46:2781–2790
Cammick EM, Veigh P, Cusker P, Timson DJ, Morphew RM, Brophy PM, Marks NJ, Mousley A, Maule A (2016) Parasit Vectors 9:46
Kisiel A, Katarzyna K, Gniadek M, Maksmiuk K, Michalska A (2015) Talanta 144:398–403
Benincasa M, Francescon M, Fregonese M, Gennaro R, Pengo P, Rossi P, Scrimin P, Tecilla P (2015) Bioorg Med Chem 23:7386–7393
Abdel Aziz AA, Seda SH, Mohammed SF (2016) Sensors Actuators B Chem 223:566–575
Xie X, Gutierrez A, Trofimov V, Szilagi I, Soldati T, Bakker E (2015) Anal Chem 87:9954–9959
Lomora M, Dinu IA, Itel F, Rigo S, Spulber M, Palivan CG (2015) Macromol Rapid Commun 36:1929–1934
Siegmann K, Stechi R, Widler R, Hirayama M (2013) J Appl Polym Sci 129:2727–2734
Vazirian MM, Charpentier TVJ, de Oliveira Penna M, Neville A (2016) J Pet Sci Eng 137:22–32
da Rosa CM, Menger RK, Gomes MT, Oliveira C (2015) Material 20:514–522
Bin M, Amer B (2015) J Pet Sci 90:124–130
Yan F, Zhang F, Bhandari N, Wang L, Dai Z, Zhang L, Liu Y, Ruan G, Kan A, Tomson M (2015) J Pet Sci 136:32–40
Yi P, Liu Z, Wang Yu X, Zhou J, Hou B, Li O (2013) Int J Quantum Chem 113:1316–1324
Pesonen H, Aksela R, Lassonen K (2010) J Phys Chem A 114:466–473
Daniele P, Poti C, Gianguzza A, Prenesti E, Sammartano S (2008) Coord Chem Ver 252:1093–1107
Pearson RG (1963) J Am Chem Soc 85:3533–3539
Despotovic I (2015) New J Chem 39:6151–6162
Kowalsak-Baron A (2015) Comput Theor Chem 1057:7–14
Calhorda MJ, Krapp A, Frenking G (2007) J Phys Chem A 111:2859–2869
Pandey KK, Patidar P (2012) J Organomet 702:59–66
Bayat M, Hopffgarten M, Salehzadeh S, Frenking G (2011) J Organomet Chem 696:2976–2984
Baskaran S, Venuvanalingam P, Silvasankar C (2011) J Organomet Chem 696:2627–2634
Morokuma K (1977) J Chem Phys 55:1236–1244
Morokuma K (1977) Acc Chem Res 10:294–300
Zieger T, Rauk A (1977) Theor Chim Acta 46:1–10
Phipps MJS, Fox T, Tautermann CS, Skylaris CK (2015) R Soc Chem 44:3177–3211
da Costa LM, Carneiro JWM, Paes LWC (2009) J Mol Struct THEOCHEM 911:46–51
da Costa LM, Carneiro JWM, Romeiro GA, Paes LWC (2011) J Mol Model 17:243–249
da Costa LM, Stoyanov SR, Damasceno RN, Carneiro JWM (2013) Int J Quantum Chem 113:2621–2628
Costa LM, Ferreira GB, Carneiro JWM (2013) J Mol Model 19:2669–2677
Costa LM, Paes LWC, Carneiro JWM (2012) J Braz Chem Soc 23:648–655
Costa LM, Stoyanov SR, Carneiro JWM (2012) J Mol Model 18:4389–4396
Costa LM, Amorim RM, Cruzm MTM, Carneiro JWM (2012) Comput Theor Chem 999:7–12
Costa LM, Carneiro JWM, Paes LWC (2011) J Mol Model 17:2061–2067
Meuser MVM, Quattrociocchi DG, Costa LM, Ferreira GB, Carneiro JWM (2015) Polyhedron 102:193–200
Quattrociocchi DGS, Ferreira GB, da Costa LM, Carneiro JWM (2016) Comput Theor Chem 1075:104–110
Andrini O, Meinild AK, Ghezzi C, Murer H, Forster IC (2012) Am J Phys Cell Phys 302:539–554
Ruan CH, Rodgers MT (2009) J Am Chem Soc 131:10918–10928
Ling BP, Yan XY, Sun M, Bi SW (2016) Comput Biol Chem 60:21–31
Becke AD (1992) J Chem Phys 96:2155–2160
Rassolov VA, Ratner MA, Pople JA, Redfern PC, Curtis LA (2001) J Comput Chem 22:976–984
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford CT
Corral I, Mo O, Yanez M, Scott A, Random L (2003) J Phys Chem A 107:10456–10461
Trujillo AM, Lamsabhi O, Yanez M, Salpin J (2008) J Org Biomol 6:3695–3702
Peschke M, Blades AT, Kebarle P (2000) J Am Chem Soc 122:10440–10449
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Simon S, Duran M, Dannenberg JJ (1996) J Chem Phys 105:11024–11031
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3094
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jense JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupious M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Dudev M, Wang J, Dudev T, Lim C (2006) J Phys Chem B 110:1889–1895
Shimzaki Y, Arai N, Dunn TJ, Yajima TM, Tani F, Ramogida CF, Storr T (2011) Dalton Trans 40:2469–2479
Coupez B, Wipff G (2003) Inorg Chem 42:3693–3703
Davydova EI, Sevastionova TN, Timoshkin AY, Suvorov AV, Frenking G (2004) Int J Quantum Chem 100:419–425
Shinzato Y, YuKawa H, Morinaga M, Baba T, Nakai H (2007) J Alloys Compd 446:96–100
Brown HC, Okamoto Y (1958) J Am Chem Soc 80:4979–4982
Duvoisi S, Lima ICV, Ighor CV, Kuhnen AC (2011) Quim Nov. 34:1595–1603
Rodgers MT, Armentrout PB (2000) Mass Spectrom Ver 19:215–247
Ruan CH, Huang H, Rodegers MT (2008) J Am Soc Mass Spectrom 19:305–314
Coupez B, Boehme C, Wipff G (2002) Phys Chem Chem Phys 4:5716–5729
Acknowledgments
L.M.da C., J.W.de M.C. and G.B.F. acknowledge FAPERJ (Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and the Computational Canada West Grid Cluster. S.R.S. thanks Dr. John M. Villegas for discussions. D.G.S.Q. acknowledges CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and Rafhaela M. Nascimento.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Highlights
The optimal number of spacer methylene groups of the ligand is 3–4 units.
The ligand affinity was measured by energetic, geometric and electronic parameters.
The electronic term was the major term for stabilization of the coordination interaction.
The polarization, dispersion and repulsion components were almost constant.
Polysubstitution showed that the stabilizing energy per water molecule is almost constant.
Bidentate carbonyl ligands showed strong stabilization by the chelate effect.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Quattrociocchi, D.G.S., Meuser, M.V.M., Ferreira, G.B. et al. A density functional theory investigation of the interaction of the tetraaqua calcium cation with bidentate carbonyl ligands. J Mol Model 23, 60 (2017). https://doi.org/10.1007/s00894-017-3240-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3240-0