Abstract
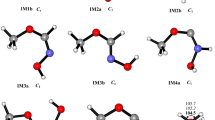
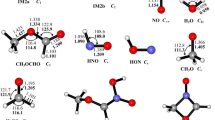
Nitromethane (NM, CH3NO2) is a widely studied energetic material, and its decomposition mechanism attracts great interest. In this work, bimolecular reactions between NO2 and nine intermediates generated during the decomposition of NM were investigated by computational chemistry methods. The mechanisms of the reactions were analyzed. The results revealed that these reactions possess small barriers and can easily occur, so they may be responsible for NO2 loss during the decomposition of NM.










Similar content being viewed by others
References
Guo F, Cheng XL, Zhang H (2012) Reactive molecular dynamics simulation of solid nitromethane impact on (010) surfaces induced and nonimpact thermal decomposition. J Phys Chem A 116:3514–3520
Guo F, Zhang H, Cheng XL (2010) Molecular dynamic simulations of solid nitromethane under high pressures. J Theor Comput Chem 9:315–325
Hu WF, He TJ, Chen DM, Liu FC (2002) Theoretical study of the CH3NO2 unimolecular decomposition potential energy surface. J Phys Chem A 106:7294–7303
Rom N, Zybin SV, van Duin ACT, Goddard WA III, Zeiri Y, Katz G, Kosloff R (2011) Density-dependent liquid nitromethane decomposition: molecular dynamics simulations based on ReaxFF. J Phys Chem A 115:10181–10202
Citroni M, Datchi F, Bini R, Vaira MD, Pruzan P (2008) Crystal structure of nitromethane up to the reaction threshold pressure. J Phys Chem B 112:1095–1103
Mueller KH (1955) The thermal decomposition of nitromethane at high pressures. J Am Chem Soc 77:3459–3462
Cottrell TL, Graham TE, Reid TJ (1950) The thermal decomposition of nitromethane. J Chem Phys 18:1306
Chang J, Lian P, Wei DQ, Cheng XR, Zhang QM, Gong ZZ (2010) Thermal decomposition of the solid phase of nitromethane: ab initio molecular dynamics simulations. Phys Rev Lett 105:188302
Margetis D, Kaxiras E, Elstner M, Frauenheim T, Manaa MR (2002) Electronic structure of solid nitromethane: effects of high pressure and molecular vacancies. J Chem Phys 117:788–799
McKee ML (1986) Ab initio study of rearrangements on the nitromethane potential energy surface. J Am Chem Soc 108:5784–5792
Manaa MR, Reed EJ, Fried LE, Galli G, Gygi F (2004) Early chemistry in hot and dense nitromethane: molecular dynamics simulations. J Chem Phys 120:10146
Zhu RS, Lin MC (2009) CH3NO2 decomposition/isomerization mechanism and product branching ratios: an ab initio chemical kinetic study. Chem Phys Lett 478:11–16
Zhu RS, Raghunath P, Lin MC (2013) Effect of roaming transition states upon product branching in the thermal decomposition of CH3NO2. J Phys Chem A 117:7308–7313
Homayoon Z, Bowman JM (2013) Quasiclassical trajectory study of CH3NO2 decomposition via roaming mediated isomerization using a global potential energy surface. J Phys Chem A 117:11665–11672
Han SP, Van Duin ACT, Goddard WA III (2011) Thermal decomposition of condensed-phase nitromethane from molecular dynamics from ReaxFF reactive dynamics. J Phys Chem B 115:6534–6540
Butler LJ, Krajnovich D, Lee YT, Ondrey GS, Bersohn R (1983) The photodissociation of nitromethane at 193 nm. J Chem Phys 79:1708–1722
Irikura KK, Johnson RD (2006) Is NO3 formed during the decomposition of nitramine explosives? J Phys Chem A 110:13974–13978
Jiang FL, Zhai GH, Ding L, Yue KF, Liu N, Shi QZ, Wen ZY (2010) Acta Phys-Chim Sin 26:409–414
Zhang JD, Kang LH, Cheng XL (2015) Theoretical study of the reaction mechanism of CH3NO2 with NO2, NO and CO: the bimolecular reactions that cannot be ignored. J Mol Model 21:13
Frisch MJ, Trucks GW, Fox DJ, et al. (2010) Gaussian 09, revision E.01. Gaussian, Inc., Wallingford
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Gutsev GL, Jena P, Bartlett RJ (1999) Thermodynamical stability of CH3ONOCH3ONO and CH3ONO− CH3ONO−: a coupled-cluster and Hartree–Fock-density-functional-theory study. J Chem Phys 110:403
Martínez-Núñez E, Borges I Jr, Vázquez SA (2002) Rate constants for the CH(3)O+NO -> CH(3)ONO reaction by classical trajectory and canonical variational transition state theory calculations. J Phys Org Chem 15:123–129
Acknowledgements
This project was supported by the National Natural Science Foundation of China (no. 21363019) and the Science and Technology Innovation Team Construction Project of Xinjiang Uygur Autonomous Region of China (no. 2014751001).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 553 kb)
Rights and permissions
About this article
Cite this article
Zhang, JD., Zhang, LL. & Cheng, XL. Theoretical studies of some bimolecular reactions during the decomposition of CH3NO2: reactions between NO2 and nine intermediates. J Mol Model 23, 62 (2017). https://doi.org/10.1007/s00894-017-3231-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3231-1