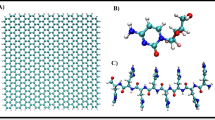
Abstract
In this work, molecular dynamics simulations are used to study the adsorption of paclitaxel (PTX) drug on the graphene-based nanomaterials including graphene (G), graphene oxide (GO), and functionalized GO with chitosan (GO-CS). The drug is adsorbed through different patterns on the surface of graphene-based nanomaterials. Our results show that PTX on graphene is adsorbed more quickly than other systems. Comparing center of mass (COM) in GO and GO-CS systems indicated that PTX approaches GO-CS surface faster than GO surface. The binding of PTX molecule to graphene surface is stronger than the other investigated systems. Our study indicated that π−π stacking and hydrophobic interactions are the main driving forces for the adsorption of the drug on graphene, while the adsorption of PTX on GO-CS is dominated by the formation of hydrogen bonds. It is found that the number of hydrogen bonds in PTX-GO-CS system is more than that of PTX−GO emphasizing the advantages of the functional group of chitosan in improving the adsorption of the drug onto nanomaterial. These results suggest that hydrogen bond, π-π stacking, and hydrophobic interactions play a key role in the adsorption of PTX in graphene-based nanomaterials. In spite of similar dimensions of investigated nanomaterials, the difference in surface chemistries and also the type of functional group can be effective factors in determining their interactions with PTX.














Similar content being viewed by others
References
Yin T, Wang L, Yin L et al (2015) Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 61:10–25
Huizing MT, Misser VS, Pieters RC et al. (1995) Taxanes: a new class of antitumor agents. Cancer Invest 13:381–404
Rowinsky EK, Cazenave LA, Donehower RC (1990) Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst 82:1247–1259
Wall ME, Wani MC (1995) Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award Lecture. Cancer Res 55:753–760
Yeh MK, Coombes AGA, Jenkins PG et al. (1995) A novel emulsification-solvent extraction technique for production of protein loaded biodegradable microparticles for vaccine and drug delivery. J Controlled Release 33:437–445
Yeung TK, Germond C, Chen X et al. (1999) The mode of action of taxol: apoptosis at low concentration and necrosis at high concentration. Biochem Biophys Res Commun 263:398–404
Feng SS, Huang G (2001) Effects of emulsifiers on the controlled release of paclitaxel (Taxol®) from nanospheres of biodegradable polymers. J Controlled Release 71:53–69
Liao PC, Lieu CH (2005) Cell cycle specific induction of apoptosis and necrosis by paclitaxel in the leukemic U937 cells. Life Sci 76:1623–1639
Brannon-Peppas L, Blanchette JO (2004) Nanoparticle and targeted systems for cancer therapy. Ad Drug Deliery Re 56:1649–1659
Singla AK, Garg A, Aggarwal D (2002) Paclitaxel and its formulations. Int J Pharm 235:179–192
Jin J, Lee WS, Joo KM et al. (2011) Preparation of blood-brain barrier-permeable paclitaxel-carrier conjugate and its chemotherapeutic activity in the mouse glioblastoma model. Med Chem Commun 2:270–273
Mathew AE, Mejillano MR, Nath JP et al. (1992) Synthesis and evaluation of some water-soluble prodrugs and derivatives of taxol with antitumor activity. J Med Chem 35:145–151
Vashist SK, Zheng D, Pastorin G et al. (2011) Delivery of drugs and biomolecules using carbon nanotubes. Carbon 49:4077–4097
Liu Z, Wang Y, Zhang N (2012) Micelle-like nanoassemblies based on polymer–drug conjugates as an emerging platform for drug delivery. Expert Opin Drug Discovery 9:805–822
Pahuja P, Arora S, Pawar P (2012) Ocular drug delivery system: a reference to natural polymers. Expert Opin Drug Discovery 9:837–861
Szűts A, Szabó-Révész P (2012) Sucrose esters as natural surfactants in drug delivery systems—a mini-review. Int J Pharm 433:1–9
Sun X, Feng Z, Hou T et al. (2014) Mechanism of graphene oxide as an enzyme inhibitor from molecular dynamics simulations. ACS Appl Mater Interfaces 6:7153–7163
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Liu F, Ming P, Li J (2007) Ab initio calculation of ideal strength and phonon instability of graphene under tension. Phys Rev B 76:064120–7
Lee C, Wei X, Kysar JW et al. (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321:385–388
He H, Klinowski J, Forster M et al. (1998) A new structural model for graphite oxide. Chem Phys Lett 287:53–56
Yang HF, Shan CS, Li FH et al. (2009) Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem Commun 14:3880–3882
Liu Z, Robinson JT, Sun X et al. (2008) PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc 130:10876–10877
Thein-Han WW, Saikhun J, Pholpramoo C et al. (2009) Chitosan–gelatin scaffolds for tissue engineering: physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP–buffalo embryonic stem cells. Acta Biomater 5:3453–3466
Thein-Han WW, Kitiyanant Y, Misra RDK (2008) Chitosan as scaffold matrix for tissue engineering. Mater Sci Technol 24:1062–1075
Rana VK, Choi MC, Kong JY et al. (2011) Synthesis and drug‐delivery behavior of chitosan‐functionalized graphene oxide hybrid nanosheets. Macromol Mater Eng 296:131–140
Bao H, Pan Y, Ping Y et al. (2011) Chitosan‐functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 7:1569–1578
Guo QF, Cao H, Li XH et al. (2015) Thermosensitive hydrogel drug delivery system containing doxorubicin loaded CS–GO nanocarriers for controlled release drug in situ. Mater Technol 30:294–300
Frisch A, Dennington RD, Keith TA et al. (2003) GaussView Version 3.0. Gaussian Inc., Pittsburgh, PA
Ricci CG, de Andrade AS, Mottin M et al. (2010) Molecular dynamics of DNA: comparison of force fields and terminal nucleotide definitions. J Phys Chem B 114:9882–9893
Berendsen HJ, Postma JV, van Gunsteren WF et al. (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N⋅ log (N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Hess B, Bekker H, Berendsen HJ et al. (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graphics 14:33–38
Zaboli M, Raissi H (2016) The influence of nicotine on pioglitazone encapsulation into carbon nanotube: the investigation of molecular dynamic and density functional theory. J Biomol Struct Dyn. doi:10.1080/07391102.2016.1152565
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
The different parameters such as the center of mass, the number of contacts, contact area, RMSD plots, radial distribution function, interaction energies between the nanomaterials, and drug molecule for first and second runs are available in supplementary information.
ESM 1
(DOCX 7016 kb)
Rights and permissions
About this article
Cite this article
Hasanzade, Z., Raissi, H. Investigation of graphene-based nanomaterial as nanocarrier for adsorption of paclitaxel anticancer drug: a molecular dynamics simulation study. J Mol Model 23, 36 (2017). https://doi.org/10.1007/s00894-017-3207-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3207-1