Abstract
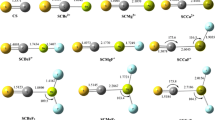
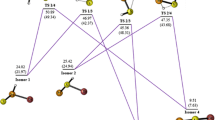
Recent ab initio investigations of some complexes formed between carbon dioxide and its analogues carbonyl sulfide, carbonyl selenide, carbon disulfide, and thiocarbonyl selenide and the common bases ammonia, water, phosphine, and hydrogen sulfide have revealed significant differences between the properties of those complexes bound through the oxygen atom of the electron acceptor and their counterparts in which the interaction takes place through a sulfur atom. In each case the interaction is weak, but the structures, interaction energies, and vibrational spectra of the complexes show some regular variations in behavior as the base and the acid are systematically changed. The adducts bound through sulfur present examples of the type of non-covalent interaction known as the chalcogen bond. In this paper we extend the range of electron acceptors to include carbon diselenide, and we explore the effects of substituting selenium for sulfur as the acceptor atom in the complexes of OCSe, SCSe, and CSe2. These adducts are also classified as chalcogen-bonded complexes, and have many features in common with the sulfur-bonded species, but also exhibit some noticeable differences between the two series.






Similar content being viewed by others
References
Rosenfield RE Jr, Parthasarathy R, Dunitz JD (1977) J Am Chem Soc 99:4860–4862
Burling FT, Goldstein BM (1992) J Am Chem Soc 114:2313–2320
Minyaev RM, Minkin VI (1998) Can J Chem 76:776–788
Iwaoka M, Takemoto S, Tomoda S (2002) J Am Chem Soc 124:10613–10620
Werz DB, Gleiter R, Rominger F (2002) J Am Chem Soc 124:10638–10639
Sanz P, Yanez M, Mo O (2002) J Phys Chem A 106:4661–4668
Sanz P, Mo O, Yanez M (2003) Phys Chem Chem Phys 5:2942–2947
Cozzolino AF, Vargas-Baca I, Mansour S, Mahmoudkhani AH (2005) J Am Chem Soc 127:3184–3190
Bleiholder C, Werz DB, Köppel H, Gleiter R (2006) J Am Chem Soc 128:2666–2674
Bleiholder C, Gleiter R, Werz DB, Köppel H (2007) Inorg Chem 46:2249–2260
Politzer P, Murray JS, Lane P (2007) Intern J Quantum Chem 107:3046–3052
Murray JS, Lane P, Clark T, Politzer P (2007) J Mol Model 13:1033–1038
Politzer P, Murray JS, Concha MC (2008) J Mol Model 14:659–665
Murray JS, Concha MC, Lane P, Hobza P, Politzer P (2008) J Mol Model 14:699–704
Murray JS, Lane P, Politzer P (2008) Intern J Quantum Chem 108:2770–2781
Choudhary A, Gandla D, Krow GR, Raines RT (2009) J Am Chem Soc 131:7244–7246
Murray JS, Lane P, Politzer P (2009) J Mol Model 15:723–729
Wang W, Ji B, Zhang Y (2009) J Phys Chem A 113:8132–8135
Shishkin OV, Omelchenko IV, Kalyuzhny AL, Paponov BV (2010) Struct Chem 21:1005–1011
Scheiner S (2011) J Chem Phys 134:164313
Junming L, Yunxiang L, Subin Y, Weiliang Z (2011) Struct Chem 22:757–763
Sanchez-Sanz G, Alkorta I, Elguero J (2011) Mol Phys 109:2543–2552
Milov AA, Minyaev RM, Minkin VI (2011) J Phys Chem A 115:12973–12982
Iwaoka M, Isozumi N (2012) Molecules 17:7266–7283
Li Q-Z, Li R, Guo P, Li H, Li W-Z, Cheng J-B (2012) Comput Theoret Chem 980:56–61
Adhikari U, Scheiner S (2012) J Phys Chem A 116:3487–3497
Manna D, Mugesh G (2012) J Am Chem Soc 134:4269–4279
Scheiner S (2013) Cryst Eng Commun 15:3119–3124
Scheiner S (2013) Accounts Chem Res 46:280–288
Scheiner S (2013) Intern J Quantum Chem 113:1609–1620
Brezgunova ME, Lieffrig J, Aubert E, Dahaoui S, Fertey P, Lebegue S, Angyan JG, Fourmigue M, Espinosa E (2013) Cryst Growth Des 13:3283–3289
Politzer P, Murray JS, Clark T (2013) Phys Chem Chem Phys 15:11178–11189
Minyaev RM, Gribanova TN, Minkin VI. Comprehensive Inorganic Chemistry. II. From Elements to Applications (2013) 9:109–132
Bauza A, Alkorta I, Frontera A, Elguero J (2013) J Chem Theory Comput 9:5201–5210
Hobza P, Müller-Dethlefs K (2010) Non-covalent interactions. Theory and experiment. RSC, Cambridge
Latimer WM, Rodebush WH (1920) J Am Chem Soc 42:1419–1433
Shigorin DN (1959) Spectrochim Acta 14:198–212
Dumas J-M, Peurichard H, Gomel M (1978) J Chem Res S:54–57
Clark T, Hennemann M, Murray JS, Politzer P (2007) J Mol Model 13:291–296
Murray JS, Riley KE, Politzer P, Clark T (2010) Aust J Chem 63:1598–1607
Ramasami P, Ford TA (2014) Mol Phys 112:683–693
Ramasami P, Ford TA (2014) J Mol Structure 1072:28–31
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian-09, Revision D.01. Gaussian Inc, Wallingford
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Dunning TH Jr (1989) J Chem Phys 90:1007–1023
Kendall RA, Dunning TH Jr, Harrison RJ (1992) J Chem Phys 96:6796–6806
Woon DE, Dunning TH Jr (1993) J Chem Phys 98:1358–1371
Peterson KA, Woon DE, Dunning TH Jr (1994) J Chem Phys 100:7410–7415
Wilson A, van Mourik T, Dunning TH Jr (1997) J Mol Structure (Theochem) 388:339–349
Liu B, McLean AD (1973) J Chem Phys 59:4557–4558
Boys SF, Bernardi F (1970) Mol Phys 19:553–556
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Shoemaker RL, Flygare WH (1970) Chem Phys Letters 6:576–578
Benkova Z, Sadlej AJ (2004) Mol Phys 102:687–699
Politzer P, Riley KE, Bulat FA, Murray JS (2012) Comput Theoret Chem 998:2–8
Bhudhun A, Ramasami P, Murray JS, Politzer P (2013) J Mol Model 19:2739–2746
Murray JS, Macaveiu L, Politzer P (2014) J Comput Sci 5:590–596
Haynes WM (ed) (2011) CRC handbook of chemistry and physics, 91st edn. CRC, Boca Raton, pp 10-189 to 10-190
Ramasami P, Ford TA (2010) J Mol Structure (Theochem) 940:50–55
Ramasami P, Ford TA (2010) Can J Chem 88:716–724
Ramasami P, Ford TA (2012) Comput Theoret Chem 990:227–235
Ramasami P, Ford TA (2012) J Mol Struct 1023:163–169
Kutzelnigg W (1984) Angew Chem Int Ed Engl 23:272–295
Bader RFW (1990) Atoms in molecules — a quantum theory. Clarendon, Oxford
Bader RFW (1991) Chem Rev 91:893–928
Acknowledgments
This work is based on research supported in part by the National Research Foundation of South Africa (NRF) under Grant No. 2053648. The grantholder acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by NRF-supported research are those of the authors and that the NRF accepts no liability in this regard. The authors also acknowledge the Universities of Mauritius and KwaZulu-Natal for financial assistance and the Centre for High Performance Computing (South Africa) for the use of computational facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramasami, P., Ford, T.A. Chalcogen-bonded complexes. Selenium-bound adducts of NH3, H2O, PH3, and H2S with OCSe, SCSe, and CSe2 . J Mol Model 21, 35 (2015). https://doi.org/10.1007/s00894-014-2562-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2562-4