Abstract
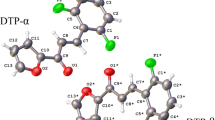
Chalcones are an important class of medicinal compounds and are known for taking part in various biological activities as in anti-inflammatory, anti-leishmania, antimitotic, and antiviral. Chemically, chalcones consist of open-chain flavonoids in which the two aromatic rings are joined by a three-carbon α,β-unsaturated carbonyl system. The wide action spectrum has attracted our attention to synthesize, crystallize, and characterize the dimethoxy-chalcone C18H18O3. Aiming to understand the process of crystal lattice stabilization, a combination of technique has been used including X-ray diffraction, infrared spectroscopy and computational molecular modeling. The theoretical calculations were carried out by the density functional method (DFT) with the M06-2X functional, with the 6-311+G(d,p) basis set. The vibrational wavenumbers were calculated and the scaled values were compared with experimental FT-IR spectrum. The intermolecular interactions were quantified and intercontacts in the crystal structure were analyzed using Hirshfeld surfaces. Bond distances and angles described by the X-ray diffraction and theoretical calculation are very similar. The C-H….O contacts contributing to assemble the supramolecular architecture are also responsible for the molecular structure assembly.







Similar content being viewed by others
References
Rao YK, Fang SH, Tzeng YM (2009) Synthesis and biological evaluation of 3′,4′,5′-trimethoxychalcone analogues as inhibitors of nitric oxide production and tumor cell proliferation. Bioorg Med Chem 17(23):7909–7914
Wu J et al. (2012) Synthesis and crystal structure of chalcones as well as on cytotoxicity and antibacterial properties. Med Chem Res 21(4):444–452
Juvale K, Pape VFS, Wiese M (2012) Investigation of chalcones and benzochalcones as inhibitors of breast cancer resistance protein. Bioorg Med Chem 20:346–355
Liu M, Wilairat P, Croft SL, Tand ALC, Goa ML (2003) Bioorg Med Chem 11:2729–2738
Trivedi JC, Bariwal JB, Upadhyay KD, Naliapara YT, Joshi SK, Pannecouque CC, Clercqd ED, Shah AK (2007) Improved and rapid synthesis of new coumarinyl chalcona derivates and their antiviral activity. Tetrahedron Lett 48:8472–8474
Bandgar BP, Gawande SS, Bodade RG, Totre JV, Khobragade CN (2010) Synthesis and biological evaluation of simple methoxylatedchalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg Med Chem 18:1364–1370
Katsori AM, Hadjipavlou-Litina D (2011) Recent progress in therapeutic applications of chalcones. Exp Opin Ther Patents 21:1575–1596. doi:10.1517/13543776.2011.596529
Edward HK, Li D (2008) Drug-like properties: concepts, structure design and methods. Elsevier, San Diego
Basavoju S, Boström D, Velaga S (2008) Indomethacin–saccharin cocrystal: design, synthesis and preliminary pharmaceutical characterization. Pharm Res 25(3):530–541
Silverman RB (1992) The organic chemistry of drug design and drug action. Elsevier, San Diego
Enraf-Nonius (1993) CAD-4 = PC 1.2 Enraf-Nonius. Delft, Netherlands
Sheldrick GM (1990) SHELXS-97 Program for the solution of crystal structures. University of Gottingen, Germany
Enraf-Nonius (2000) COLLECT Nonius BV. Delft, Netherlands
Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. In: Carter CWJ (ed) Methods in enzymology, vol 287. Academic, San Diego, pp 307–326
Farrugia L (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Crystallogr 32(4):837–838
Farrugia L (1997) ORTEP-3 for Windows - a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Crystallogr 30:565–1997
Macrae CF et al (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39(3):453–457
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. Cryst Eng Comm 4(66):378–392
McKinnon JJ, Spackman MA, Mitchell AS (2004) Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr B 60(6):627–668
Wolff SK et al (2007) Crystal Explorer 2.1. University of Western Australia, Perth, Australia
Frisch MJ et al. (2004) Gaussian 03 Revision C02. Gaussian Inc, Wallingford, CT
Frisch MJ et al (2009) Gaussian Inc. Wallingford, CT
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Account 120:215–241
GaussView, Version 5, Dennington R, Keith T, Millam J (2009) Semichem Inc, Shawnee Mission
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650
Frisch MJ, Pople AJ, Binkley JS (1984) J Chem Phys 80:3265
Politzer P, Murray JS (2002) Theor Chem Acc 108:134
Scott AP, Radom L (1996) J Phys Chem 100:16503–16513
Russell D. Johnson III, (2013), NIST computational chemistry comparison and benchmark database. IOP Publishing web. http://cccbdb.nist.gov/vibscale2.asp?method=58&basis=1. Accessed 26 Mar 2014
Silverstein M, Basseler GC, Morill C (1981) Spectrometric identification of organic compounds. Wiley, New York
Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG (1991) The handbook of infrared and Raman characteristic frequencies of organic molecules. Academic, Boston
Pearson RG (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci U S A 83:8440–8441
Sklenar H, Jager J (1979) Molecular structure-biological activity relationships on the basis of quantum-chemical calculations. Int J Quantum Chem 26:467–484
Zhow Z, Parr RG (1990) Activation hardness: New index for describing the orientation of electrophilic aromatic substitution. J Am Chem Soc 112:5720–5724
Fukui K (1982) Science 218:747–754
Kosar B, Albayrak C (2011) Spectrochim Acta A 78:160–167
Singh UC, Kollman PA (1984) An approach to computing electrostatic charges for molecules. J Comput Chem 5(2):129–145
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J Comput Chem 11(3):361–373
Acknowledgments
The authors would like to acknowledge the following Brazilian agencies for financial support: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo Pesquisa do Estado de Goiás (FAPEG).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection Brazilian Symposium of Theoretical Chemistry (SBQT2013)
Rights and permissions
About this article
Cite this article
Ternavisk, R.R., Camargo, A.J., Machado, F.B.C. et al. Synthesis, characterization, and computational study of a new dimethoxy-chalcone. J Mol Model 20, 2526 (2014). https://doi.org/10.1007/s00894-014-2526-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2526-8