Abstract
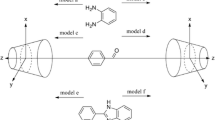
The synthesis of 2-aryl-2,3-dihydro-4-quinolones in the presence of per-6-amino-β-cyclodextrin (per-6-ABCD) as catalyst can improve selectivity and yield. The interaction between per-6-ABCD and benzaldehyde or o-aminoacetophenone plays an important role in this reaction. This paper studies the complexes of per-6-ABCD with benzaldehyde and o-aminoacetophenone using density functional theory (DFT) method. The reaction process is investigated by studying the energy of the reactants and the product. Hydrogen bonds are researched on the basis of natural bonding orbital (NBO) analysis, the results propose the donor–acceptor interactions of complex. The Mulliken charge and frontier orbital are employed for revealing the charge distribution. In addition, 13C nuclear magnetic resonance (13CNMR) spectroscopy shows that the carbon atom on the aldehyde group for benzaldehyde, carbonyl group and the carbon atom connected with carbonyl group for o-aminoacetophenone are apparently activated in the cavity of per-6-ABCD. The probable catalytic mechanism of per-6-ABCD is discussed in terms of the calculated parameters.

Per-6-amino-β-cyclodextrin as catalyst in synthesis of 2-aryl-2,3-dihydro-4-quinolones



Similar content being viewed by others
References
Zhang J, Ma PX (2013) Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev 65:1215–1233
Hasegawa Y, Inoue Y, Deguchi K, Ohki S, Tansho M, Shimizu T, Yazawa K (2012) Molecular dynamics of a polyaniline/β-cyclodextrin complex investigated by 13C solid-state NMR. J Phys Chem B 116:1758–1764
Li Z, Couzijn EPA, Zhang X (2012) Intrinsic properties of α-cyclodextrin complexes with benzoate derivatives in the gas phase: an experimental and theoretical study. J Phys Chem B 116:943–950
Dsouza RN, Pischel U, Nau WM (2011) Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem Rev 111:7941–7980
López CA, de Vries AH, Marrink SJ (2013) Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Sci Rep-UK 3:2071
Nakamura A, Inoue Y (2005) Electrostatic manipulation of enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate within γ-cyclodextrin cavity through chemical modification. inverted product distribution and enhanced enantioselectivity. J Am Chem Soc 127:5338–5339
Del Valle EM (2004) Cyclodextrins and their uses: a review. Process Biochem 39:1033–1046
Ji H, Huang L, Shen H, Zhou X (2011) β-Cyclodextrin-promoted synthesis of 2-phenylbenzimidazole in water using air as an oxidant. Chem Eng J 167:349–354
Shin JA, Lim YG, Lee KH (2012) Copper-catalyzed azide − alkyne cycloaddition reaction in water using cyclodextrin as a phase transfer catalyst. J Org Chem 77:4117–4122
Breslow R, Dong SD (1998) Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem Rev 98:1997–2011
Bellia F, La Mendola D, Pedone C, Rizzarelli E, Saviano M, Vecchio G (2009) Selectively functionalized cyclodextrins and their metal complexes. Chem Soc Rev 38:2756–2781
Marchetti L, Levine M (2011) Biomimetic catalysis. ACS Catal 1:1090–1118
Liu Y, Han BH, Sun S-X, Wada T, Inoue Y (1999) Molecular recognition study on supramolecular systems. 20. Molecular recognition and enantioselectivity of aliphatic alcohols by L-tryptophan-modified β-cyclodextrin. J Org Chem 64:1487–1493
Chan W-K, Yu W-Y, Che C-M, Wong M-K (2003) A cyclodextrin-modified ketoester for stereoselective epoxidation of alkenes. J Org Chem 68:6576–6582
Kieser B, Fietzek C, Schmidt R, Belge G, Weimar U, Schurig V, Gauglitz G (2002) Use of a modified cyclodextrin host for the enantioselective detection of a halogenated diether as chiral guest via optical and electrical transducers. Anal Chem 74:3005–3012
Jones CP, Anderson KW, Buchwald SL (2007) Sequential Cu-catalyzed amidation-base-mediated camps cyclization: a two-step synthesis of 2-aryl-4-quinolones from o-halophenones. J Org Chem 72:7968–7973
Morales-Cid G, Fekete A, Simonet BM, Lehmann R, Cárdenas S, Zhang X, Valca´rcel M, Schmitt-Kopplin P (2010) In situ synthesis of magnetic multiwalled carbon nanotube composites for the clean-up of (fluoro) quinolones from human plasma prior to ultrahigh pressure liquid chromatography analysis. Anal Chem 82:2743–2752
Tadd AC, Matsuno A, Fielding MR, Willis MC (2009) Cascade palladium-catalyzed alkenyl aminocarbonylation/ intramolecular aryl amidation: an annulative synthesis of 2-quinolones. Org Lett 11:583–586
Lei B-L, Ding C-H, Yang X-F, Wan X-L, Hou X-L (2009) Kinetic resolution of 2,3-dihydro-2-substituted 4-quinolones by palladium-catalyzed asymmetric allylic alkylation. J Am Chem Soc 131:18250–18251
Kanagaraj K, Pitchumani K (2013) Per-6-amino-β-cyclodextrin as a chiral base catalyst promoting one pot asymmetric synthesis of 2-aryl-2,3-dihydro-4-quinolones. J Org Chem 78:744–751
Bensouilah N, Abdaoui M (2012) Inclusion complex of N-nitroso, N-(2-chloroethyl), N´, N´-dibenzylsulfamid with β-cyclodextrin: fluorescence and molecular modeling. CR Chim 15:1022–1036
Arun KT, Jayaram DT, Avirah RR, Ramaiah D (2011) β-cyclodextrin as a photosensitizer carrier: effect on photophysical properties and chemical reactivity of squaraine dyes. J Phys Chem B 115:7122–7128
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB. Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03 Revision B.05. Gaussian Inc, Pittsburgh, PA
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104–15423
Helgaker T, Wilson PJ, Amos RD, Handy NC (2000) Nuclear shielding constants by density functional theory with gauge including atomic orbitals. J Chem Phys 113:2983–2989
Olah GA, Heiner T, Rasul G, Prakash GKS (1998) 1H, 13C, 15 N NMR and theoretical study of protonated carbamic acids and related compounds. J Org Chem 63:7993–7998
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. Wires Coumpt Mol Sci 2:1–42
Desiraju GR (1996) The C − H · · · O hydrogen bond: structural implications and supramolecular design. Accounts Chem Res 29:441–449
Chocholoušová J, Špirko V, Hobza P (2004) First local minimum of the formic acid dimer exhibits simultaneously red-shifted O–H · · · O and improper blue-shifted C − H · · · O hydrogen bonds. Phys Chem Chem Phys 6:37–41
Uccello-Barretta G, Balzano F, Sicoli G, Frı́glola C, Aldana I, Monge A, Paolino D, Guccione S (2004) Combining NMR and molecular modelling in a drug delivery context: investigation of the multi-mode inclusion of a new NPY-5 antagonist bromobenzenesulfonamide into β-cyclodextrin. Bioorg Med Chem 12:447–458
Prabhu AAM, Sankaranarayanan RK, Venkatesh G, Rajendiran N (2012) Dual fluorescence of fast blue rr and fast violet B: effects of solvents and cyclodextrin complexation. J Phys Chem B 116:9061–9074
Yan Z, Zuo Z, Lv X, Li Z, Li Z, Huang W (2012) Adsorption of NO on MoO3 (0 1 0) surface with different location of terminal oxygen vacancy defects: A density functional theory study. Appl Surf Sci 258:3163–3167
Acknowledgment
The authors wish to acknowledge the financial supports from the Scientific Research Fund of Hunan Provincial Education Department (No. 12A132) for the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wan, Y., Wang, X. & Liu, N. DFT study of the per-6-amino-β-cyclodextrin as catalyst in synthesis of 2-aryl-2,3-dihydro-4-quinolones. J Mol Model 20, 2431 (2014). https://doi.org/10.1007/s00894-014-2431-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2431-1