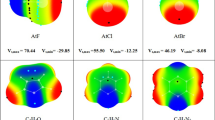
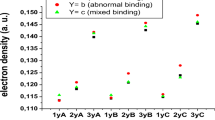
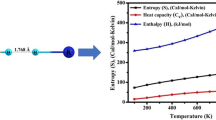
Abstract
The cooperativity effects between H-bonding and Na+⋯π or Na+⋯σ interactions in Na+⋯benzonitrile⋯H2O complexes were investigated using the B3LYP and MP2(full) methods with 6-311++G(2d,p) and aug-cc-pVTZ basis sets. The thermodynamic cooperativity and the influence of this cooperativity on aromaticity was evaluated by nucleus-independent chemical shifts (NICS). The results showed that the influence of the Na+⋯σ or Na+⋯π interaction on the hydrogen bond is more pronounced than that of the latter on the former. The cooperativity effect appeared in the Na+⋯σ interaction complex while the anti-cooperativity effect tended to be in the Na+⋯π system. The change in enthalpy is the major factor driving cooperativity. Thermodynamic cooperativity is not in accordance with the cooperativity effect evaluated by the change of interaction energy. The ring aromaticity of is weakened while the bond dissociation energy (BDE) of the C–CN bond increases upon ternary complex formation. The cooperativity effect (E coop) correlates with R c (NICS(1)ternary/NICS(1)binary) and ΔΔδ (Δδ ternary − Δδ binary) involving the ring and C ≡ N bond, as well as R BDE(C–CN) [BDE(C–CN)ternary/BDE(C–CN)binary], respectively. AIM (atoms in molecules) analysis confirms the existence of cooperativity.

Probing the influences of cooperativity on aromaticity and thermodynamic properties







Similar content being viewed by others
References
Vijay D, Sastry GN (2010) The cooperativity of cation-π and π-π interactions. Chem Phys Lett 485:235–242
Meyer EA, Castellano RK, Diederich F (2003) Interactions with aromatic rings in chemical and biological recognition. Angew Chem Int Ed 42:1210–1250
Hesselmann A, Jansen G, Schutz M (2006) Interaction energy contributions of H-bonded and stacked structures of the AT and GC DNA base pairs from the combined density functional theory and intermolecular perturbation theory approach. J Am Chem Soc 128:11730–11731
Leist R, Frey JA, Ottiger P, Frey H-M, Leutwyler S, Bachorz RA, Klopper W (2007) Nucleobase-fluorobenzene interactions: hydrogen bonding wins over π-stacking. Angew Chem Int Ed 46:7449–7452
Garcia-Raso A, Albertí FM, Fiol JJ, Tasada A, Barceló-Oliver M, Molins E, Escudero D, Frontera A, Quiñonero D, Deyà PM (2007) Anion-π interactions in bisadenine derivatives: a combined crystallographic and theoretical study. Inorg Chem 46:10724–10735
Tielrooij KJ, Garcia-Araez N, Bonn M, Bakker HJ (2010) Cooperativity in ion hydration. Science 328:1006–1009
Ebrahimi A, Habibi-Khorassani M, Gholipour AR, Masoodi HR (2009) Interaction between uracil nucleobase and phenylalanine amino acid: the role of sodium cation in stacking. Theor Chem Acc 124:115–122
Alkorta I, Blanco F, Deyà PM, Elguero J, Estarellas C, Frontera A, Quiñonero D (2010) Cooperativity in multiple unusual weak bonds. Theor Chem Acta 126:1–14
Escudero D, Frontera A, Quiñonero D, Deyà PM (2008) Interplay between cation-π and hydrogen bonding interactions. Chem Phys Lett 456:257–261
Estarellas C, Frontera A, Quiñonero D, Deyà PM (2009) Interplay between cation-π and hydrogen bonding interactions: are non-additivity effects additive? Chem Phys Lett 479:316–320
Vijay D, Zipse H, Sastry GN (2008) On the cooperativity of cation-π and hydrogen bonding interactions. J Phys Chem B 112:8863–8867
Li Q, Li W, Cheng J, Gong B, Sun J (2008) Effect of methyl group on the cooperativity between cation-π interaction and NH⋯O hydrogen bonding. J Mol Struct (THEOCHEM) 867:107–110
Wu R, McMahon TB (2008) Investigation of cation-π interactions in biological systems. J Am Chem Soc 130:12554–12556
Biot C, Wintjens R, Rooman M (2004) Stair motifs at protein-DNA interfaces: nonadditivity of H-bond, stacking, and cation-π interactions. J Am Chem Soc 126:6220–6221
Kleeberg H, Klein D, Luck WAP (1987) Quantitative infrared spectroscopic investigations of hydrogen-bond cooperativity. J Phys Chem 91:3200–3203
Ojamäe L, Hermansson K (1994) Ab initio study of cooperativity in water chains: binding energies and anharmonic frequencies. J Phys Chem 98:4271–4282
Wu YD, Zhao YL (2001) A theoretical study on the origin of cooperativity in the formation of 310− and α-Helices. J Am Chem Soc 123:5313–5319
Kar T, Scheiner S (2004) Comparison of cooperativity in CH⋯O and OH⋯O hydrogen bonds. J Phys Chem A 108:9161–9168
Cera ED (1998) Site-specific thermodynamics: understanding cooperativity in molecular recognition. Chem Rev 98:1563–1591
Behrouzi R, Roh JH, Kilburn D, Briber RM, Woodson SA (2012) Cooperative tertiary interaction network guides RNA folding. Cell 149:348–357
Whitesides GM, Krishnamurthy VM (2005) Designing ligands to bind proteins. Q Rev Biophys 38:385–395
Williams DH, Stephens E, O’Brien DP, Zhou M (2004) Understanding non-covalent interactions: ligand binding energy and catalytic efficiency from ligand-induced reductions in motion within receptors and enzymes. Angew Chem Int Ed 43:6596–6616
Birdsall B, Burgen ASV, Roberts GCK (1980) Binding of coenzyme analogs to Lactobacillus casei dihydrofolate reductase: binary and ternary complexes. Biochemistry 19:3723–3731
James F, Berry B, Nadezhda VK, Yegor DS, Emna MNP, Vladimir IP (2011) NMR structures of Apo L. Casei dihydrofolate reductase and its complexes with trimethoprim and NADPH: contributions to positive cooperative binding from ligand-induced refolding, conformational changes, and interligand hydrophobic interactions. Biochemistry 50:3609–3620
Jencks WP (1981) On the attribution and additivity of binding energies. Proc Natl Acad Sci USA 78:4046–4050
Andrea F, Jack G (2012) Enthalpy–entropy compensation and cooperativity as thermodynamic epiphenomena of structural flexibility in ligand–receptor interactions. J Mol Biol 417:454–467
Gupta RB, Brinkley RL (1998) Hydrogen-bond cooperativity in 1-alkanol + n-alkane binary mixtures. AIChE J 44:207–213
Kříž J, Dybal J (2007) Cooperative hydrogen bonds of macromolecules. 3. A model study of the proximity effect. J Phys Chem B 111:6118–6126
von Ragué SP, Manoharan M, Wang ZX, Kiran B, Jiao H, Puchta R, van Eikema Hommes NJR (2001) Dissected nucleus-independent chemical shift analysis of π-aromaticity and antiaromaticity. Org Lett 3:2465–2468
Esrafili MD, Alizadeh V (2011) Characterization of O–H⋯O interactions in linear and cyclic clusters of boric acid: An ab initio, DFT, QTAIM and NBO study. Comput Theor Chem 974:66–75
Solimannejad M, Malekani M (2012) Cooperative and diminutive interplay between the hydrogen bonding and halogen bonding in ternary complexes of HCCX (X = Cl, Br) with HCN and HNC. Comput Theor Chem 998:34–38
van Mourik T, Dingley AJ (2007) Characterizing the cooperativity in H-bonded amino structures. J Phys Chem A 111:11350–11358
Aschaffenburg DJ, Moog RS (2009) Probing hydrogen bonding environments: solvatochromic effects on the CN vibration of benzonitrile. J Phys Chem B 113:12736–12743
Kryachko ES, Nguyen MT (2001) Hydrogen bonding in benzonitrile-water complexes. J Chem Phys 115:833–841
Attah IK, Hamid AM, Meot-Ner (Mautner) M, El-Shall MS (2013) Substituent effects on noncovalent bonds: complexes of ionized benzene derivatives with hydrogen cyanide. J Phys Chem A 117:10588–10597
Heßelmann A, Jansen G, Schütz M (2005) Density-functional theory-symmetry-adapted intermolecular perturbation theory with density fitting: A new efficient method to study intermolecular interaction energies. J Chem Phys 122:014103-1–014103-17
Poovathinthodiyil R, Scott LW (2002) Cooperative C-H⋯O hydrogen bonding in CO2-lewis base complexes: implications for solvation in supercritical CO2. J Am Chem Soc 124:12590–12599
Wang Z, Zhang J, Wu J, Cao W (2007) Theoretical investigation on intermolecular interactions between HCN and HNC: the nature and thermodynamic properties. J Mol Struct (THEOCHEM) 806:239–246
Chakree T, Adam MD, Stephen GK (2008) The rotational spectrum and structure for the argon-cyclopentadienyl thallium van der Waals complex: Experimental and computational studies of noncovalent bonding in an organometallic π-complex. J Chem Phys 129:054305-1–054305-8
Alkorta I, Elguero J, Del Bene JE (2010) An ab initio investigation of the properties of H2:HX hydrogen-bonded complexes. Chem Phys Lett 489:159–163
Kendall RA Jr, Dunning TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796–6806
Frisch MJ et al., Gaussian 03, Revision B.03, Gaussian, Inc., Pittsburgh PA, 2003
Duijineveldt FB, Duijineveldt-van de Rijdt JCMV, Lenthe JHV (1994) State of the art in counterpoise theory. Chem Rev 94:1873–1885
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the difference of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Feng G, Ting-ye Qi T, Shi W, Guo Y, Zhang Y, Guo J, Kang L (2014) A B3LYP and MP2(full) theoretical investigation on the cooperativity effect between hydrogen-bonding and cation-molecule interactions and thermodynamic property in the 1: 2 (Na+: N-(Hydroxymethyl)acetamide) ternary complex. J Mol Model 20:2154. doi:10.1007/s00894-014-2154-3
Glendening ED (2005) Natural energy decomposition analysis: extension to density functional methods and analysis of cooperative effects in water clusters. J Phys Chem A 109:11936–11940
Bader RFW (1990) Atoms in molecules, a quantum theory. Oxford University Press, Oxford, UK
Wolinski K, Hilton JF, Pulay P (1990) Efficient implementation of the gauge independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc 112:8251–8260
Brink T, Haeberlin M, Jonsson M (1997) A computational analysis of substituent effects on the O−H bond dissociation energy in phenols: polar versus radical effects. J Am Chem Soc 119:4239–4244
Barckholtz C, Barckholtz TA, Hadad CM (1999) C−H and N−H bond dissociation energies of small aromatic hydrocarbons. J Am Chem Soc 121:491–500
Williams DH, Stephens E, Zhou M (2003) Ligand binding energy and catalytic efficiency from improved packing with receptors and enzymes. J Mol Biol 329:389–399
Calderone CT, Williams DH (2001) An enthalpic component in cooperativity: the relationship between enthalpy, entropy, and noncovalent structure in weak associations. J Am Chem Soc 123:6262–6267
Nasief NN, Tan H, Kong J, Hangauer D (2012) Water mediated ligand functional group cooperativity: the contribution of a methyl group to binding affinity is enhanced by a COO-group through changes in the structure and thermodynamics of the hydration waters of ligand-thermolysin complexes. J Med Chem 55:8283–8302
Author information
Authors and Affiliations
Corresponding author
Appendix A. Supplementary data
Below is the link to the electronic supplementary material.
894_2014_2341_MOESM1_ESM.doc
Some geometrical parameters of the binary and ternary complexes, and the interaction energies of the binary systems are given in this Appendix. (DOC 2317 kb)
Rights and permissions
About this article
Cite this article
Zhao, Gm., Liu, Yc., Shi, Wj. et al. A B3LYP and MP2(full) theoretical investigation into cooperativity effects, aromaticity and thermodynamic properties in the Na+⋯benzonitrile⋯H2O ternary complex. J Mol Model 20, 2341 (2014). https://doi.org/10.1007/s00894-014-2341-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2341-2