Abstract
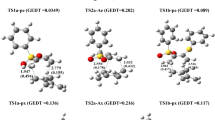
The electronic structure, the origin of the extraordinary stability and the reaction mechanisms of the decomposition reaction of the three-membered ring cyclopropenone (IO), its phenyl derivative (IIO) and its sulfur analogues (IS and IIS) have been investigated at the B3LYP/6-311 + G** level of theory. All critical points on the reaction surface, reactants, transition states and intermediates were determined. Reaction rate constants and half-lives have been computed. Natural bond orbital (NBO) analysis has been used to investigate the type and extent of interaction in the studied species. Results indicate that the decomposition reaction occurs via a stepwise mechanism, with the formation of a short-lived intermediate. The characters of the intermediates for the decomposition of IIO and IIS are different. In case of IIO decomposition, the intermediate structure is of prevailing zwitterionic character, whereas that for the decomposition of IIS is of prevailing carbene character. Solvent effects are computed, analyzed and discussed.














Similar content being viewed by others
References
Breslow R, Ryan G (1967) J Am Chem Soc 89:3073
Breslow R, Ryan G, Groves JT (1970) J Am Chem Soc 92:988
Breslow R, Oda M (1972) J Am Chem Soc 94:4787
Benson RC, Flygare WH, Oda M, Breslow R (1973) J Am Chem Soc 2772, and references therein
Oda M, Breslow R, Pecoraro J (1972) Tetrahedron Lett 4419
Breslow R, Oda M, Pecoraro J (1972) Tetrahedron Lett 4415
Yang J, McCann K, Laane J (2004) J Mol Struct 695–696:339–343
Brown FR, Finseth DH, Miller FA, Rhee KH (1975) J Am Chem Soc 97:1011
Tuazon EC, Finseth DH, Miller FA (1975) Spectrochim Acta 31A:1133
Benson RC, Flygare WH, Oda M, Breslow R (1973) J Am Chem Soc 95:2772
Komatsu K, Kitagawa T (2003) Chem Rev 103:1371
Poloukhtine A, Popik V (2003) J Org Chem 68:7833
Breslow R, Eischer T, Krebs A, Peterson RA, Posner J (1965) J Am Chem Soc 87:1320
Chiang Y, Kresge AJ, Popik V (1999) J Am Chem Soc 121:5930
Chiang Y, Kresge AJ, Paine SW, Popik V (1996) J Phys Org Chem 9:361
Wadsworth DH, Donatelliv BA (1981) Synthesis 285
Nguyen LT, DeProft F, Nguyen MT, Geerlings P (2001) J Chem Soc PerkinTrans 2:898
Poloukhtine A, Popik V (2006) J Phys Chem A110:1749
Eckart U, Sadlej AJ, Ingamells VE, Papadopoulos MG (2001) J Chem Phys 114
Breslow R, Altman LJ, Krebs A, Mohacsi E, Murata I, Peterson RA, Posner J (1965) J Am Chem Soc 87:1326
Dailey WP (1995) J Org Chem 60:6737
Andraos J, Chiang Y, Grant AS, Guo H-X, Kresge AJ (1994) J Am Chem Soc 116:7411
Chiang Y, Grant AS, Kresge AJ, Paine SW (1996) J Am Chem Soc 118:4366
Chiang Y, Grant AS, Guo H-X, Kresge AJ, Paine SW (1997) J Org Chem 62:5363
Chiang Y, Kresge AJ, Hochstrasser R, Wirz J (1989) J Am Chem Soc 111:2355
Chiang Y, Kresge AJ, Popik VV (1995) J Am Chem Soc 117:9165
Sung K, Fang D, Glenn D, Tidwell TT (1998) J Chem Soc Perkin Trans 2:2073
Frish MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG et al. (2008) Gaussian, Pittsburgh, PA
Zhang Q, Li Z, Chen B (2009) J Mol Struct (THEOCHEM) 901:202
Zhang Q, Chen B (2010) J Mol Struct (THEOCHEM) 941:10
Smith DM, Nicolaides A, Golding BT, Radom L (1998) J Am Chem Soc 120:10223
Hehre WJ, Radom L, Schleyer PVR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Lin L, Ding W-J, Fang W-H, Liu R-Z (2006) J Phys Chem A 110:8744–8749
Rochart WV, Gard GL (1969) J Org Chem 34:4173–4176
Ulic SE, Della Védova CO, Hermann A, Mack H-G, Oberhammer H (2008) J Phys Chem A 112:6211–6216
Reed AE, Weinhold F (1983) J Phys Chem 78:4066–4073
Chen XJ, Wu F, Yan M, Li HB, Tian SX, Shan X, Wang KD, Li ZJ, Xu KJ (2009) Chem Phys Lett 472(19):243–247
Frisch AE, Hratchian HP, Dennington RD II et al (2009) GaussView, Version 5.0.8. Gaussian, Wallingford
Elroby SAK, Osman OI, Aziz SG (2011) Mol Phys 109:1785–1795
Steinfeld JI, Francisco JS, Hase WL (1989) Chemical kinetics and dynamics, 2nd edn. Prentice Hall, Upper Saddle River, pp 300–301
Acknowledgments
This Project was funded by the Deanship of Scientific Research (DSR) King Abdulaziz University, Jeddah, under grant no. D19-130-/1432. The authors, therefore, acknowledge with thanks DSR support for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elroby, S.A.K., Aziz, S.G. & Hilal, R. Electronic structure and decomposition reaction mechanism of cyclopropenone, phenylcylopropenone and their sulfur analogues: a theoretical study. J Mol Model 19, 1339–1353 (2013). https://doi.org/10.1007/s00894-012-1669-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1669-8