Abstract
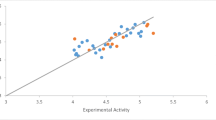
Isatin is an important compound from the biological aspect of view. It is an endogenous substance and moreover; various pharmacological activities have been reported for isatin and its derivatives. In-vitro cytotoxic effects of the prepared isatin Schiff bases toward HeLa, LS180 and Raji human cancer cell lines has been reported in our previous work. 3-(2-(4-nitrophenyl)hydrazono) indolin-2-one was found to be the most potent one among the studied compounds (IC30 = 12.2 and 21.8 μM in HeLa and LS-180 cell lines, respectively). Obtained biological data could be well interpreted using docking binding energies toward vascular endothelial growth factor receptor (VEGFR-2); a key anticancer target being biologically investigated against various isatin derivatives. In the present work, quantum mechanical (QM) method including functional B3LYP in association with split valence basis set using polarization functions (Def2-SVP) was used to estimate individual ligand-residue interaction energies for the docked 3-(2-(4-nitrophenyl)hydrazono) indolin-2-one into VEGFR-2 active site. Results were further interpreted via calculated polarization effects induced by individual amino acids of the receptor active site. A fairly good correlation could be found between polarization effects and estimated binding energies (R2 = 0.7227). Conformational analysis revealed that 3-(2-(4-nitrophenyl) hydrazono) indolin-2-one might not necessarily interact with the VEGFR-2 active site in its minimum energy conformation.

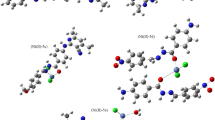
Various interactions of a 3-(2-(4-nitrophenyl) hydrazono) indolin-2-one structure with VEGFR-2 active site have been evaluated in terms of individual ligand-residue binding energies using functional B3LYP in association with Def2-SVP basis set





Similar content being viewed by others
References
Guo YC, Zhongcaoyao F (1986) TLC-UV-spectrophotometric and TLCscanning determination of isatin in leaf of Isatis. Zhongcaoyao 17:8–11
Ischia MP, Palumba A, Prota G (1988) Adrenalin oxidation revisited. New products beyond the adrenochrome stage. Tetrahedron 44(20):6441–6446
Binda C, Li M, Hubálek F, Restelli N, Edmondson DE, Mattevi A (2003) Insights into the mode of inhibition of human mitochondrial monoamine oxidase B from high-resolution crystal structures. Proc Natl Acad Sci USA 100:9750–9755
Van der Walta EM, Milczekb EM, Malana SF, Edmondsonb DE, Castagnoli N Jr, Bergha JJ, Petzer JP (2009) Inhibition of monoamine oxidase by (E)-styrylisatin analogues. Bioorg Med Chem Lett 19:2509–2513
Imam SAV, Varma RS (1975) Isatin-3-anils as excystment and cysticidal agents against Schizopyrenus russelli. Experientia 31(11):1287–1288
Varma RS, Khan IA (1977) Potential biologically active agents. X. Synthesis of 3-arylimino-2-indolinones, and their 1-methyl- and 1-morpholino/piperidinomethyl derivatives as excystment and cysticidal agents against Schizopyrenus russelli. Pol J Pharmacol Pharm 29(5):549–554
Khan KM, Khan M, Ali M, Taha M, Rasheed S, Perveen S, Choudhary MI (2009) Synthesis of bis-Schiff bases of isatins and their antiglycation activity. Bioorg Med Chem 17:7795–7801
Verma M, Pandeya SN, Singh KN, Stables JP (2004) Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharm Turc 54:49–56
Smitha S, Pandeya SN, Stables JP, Ganapathy S (2008) Anticonvulsant and sedative-hypnotic activities of N-acetyl / methyl isatin derivatives. Sci Pharm 76:621–636
Bhattacharya SK, Chakrabarti S (1998) Dose-related proconvulsant and anticonvulsant activity of isatin, a putative biological factor in rats. Indian J Exp Biol 36:118–121
Chohan ZH, Pervez H, Rauf A, Khan KM, Supuran CT (2004) Isatin-derived antibacterial and antifungal compounds and their transition metal complexes. J Enzym Inhib Med Chem 19:417–423
Shuttleworth SJN, Gervais DC, Siddiqui MA, Rando RF, Lee N (2000) Parallel synthesis of isatin-based serine protease inhibitors. Bioorg Med Chem Lett 10(22):2501–2504
Anderson A (2012) Structure-based functional design of drugs: from target to lead compound. Method Mol Biol 823:359–366
Ohacek R, McMartin C, Guida W (1996) The art and practice of structure-based drug design: a molecular modeling perspective. Med Res Rev 16:3–50
Leach A (1996) Molecular modelling. Principles and applications, 3rd edn. Addison Wesley, Boston
Vedani A, Zbinden P, Snyder J, Greenidge P (1995) Pseudoreceptor modeling: the construction of three-dimensional receptor surrogates. J Am Chem Soc 117:4987–4994
Bissantz C, Folkers G, Rognan D (2000) Protein-based virtual screening of chemical databases. 1. Evaluation of different docking/scoring combinations. J Med Chem 43:4759–4767
Kubinyi H (1997) QSAR and 3D QSAR in drug design Part 1: methodology. Drug Discov Today 2:457–467
Neese F (2011) ORCA – an ab initio, density functional and semiempirical program package. Version 2.8.0, edn. University of Bonn
Remko M, Bohác A, Kováčikova L (2011) Molecular structure, pKa, lipophilicity, solubility, absorption, polar surface area, and blood brain barrier penetration of some antiangiogenic agents. Struct Chem 22:635–648
Matesic L, Locke JM, Bremner JB, Pyne SG, Skropeta D, Ranson M, Vine KL (2008) N-phenethyl and N-naphthylmethyl isatins and analogues as in vitro cytotoxic agents. Bioorg Med Chem 16:3118–3124
Hossain MM, Islam N, Khan R, Islam M (2008) Cytotoxicity study of dimethylisatin and its heterocyclic derivatives. Bangladesh J Pharmacol 2:66–70
Vine KL, Locke JM, Ranson M, Pyne SG, Bremner JB (2007) In vitro cytotoxicity evaluation of some substituted isatin derivatives. Bioorg Med Chem 15:931–938
Pervez H, Ramzan M, Yaqub M, Khan KM (2011) Synthesis, cytotoxic and phytotoxic effects of some new N4-aryl substituted isatin-3-thiosemicarbazones. Lett Drug Des Discov 8:452–458
Sun L, Tran N, Liang C, Tang F, Rice A, Schreck R, Waltz K, Shawver LK, McMahon G, Tang C (1999) Design, synthesis, and evaluations of substituted 3-[(3- or 4-carboxyethylpyrrol-2-yl) methylidenyl] indolin-2-ones as inhibitors of VEGF, FGF, and PDGF receptor tyrosine kinases. J Med Chem 42:5120–5130
Phosrithong N, Ungwitayatorn J (2010) Molecular docking study on anticancer activity of plant-derived natural products. Med Chem Res 19:817–835
Strawn LM, McMahon G, App H, Schreck R, Kuchler WR, Longhi MP, Hui TH, Tang C, Levitzki A, Gazit A (1996) Flk-1 as a target for tumor growth inhibition. Cancer Res 56:3540–3545
Holmes K, Roberts OL, Thomas AM, Cross MJ (2007) Vascular endothelial growth factor receptor-2: structure, function, intracellular signaling and therapeutic inhibition. Cell Signal 19:2003–2012
Azizian J, Mohammadi MK, Firuzi O, Razzaghi-asl N, Miri R (2011) Synthesis, biological activity and docking study of some new isatin Schiff base derivatives. Med Chem Res accepted for publication
Schafer A, Horn H, Ahlrichs R (1992) Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J Chem Phys 97:2571–2577
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Wallace AC, Laskowski RA, Thornton JM (1995) Ligplot—a program to generate schematic diagrams of protein ligand interactions. Protein Eng 8:127–134
Fogarasi G, Zhou X, Taylor PW, Pulay P (1992) The calculation of ab initio molecular geometries: efficient optimization by natural internal coordinates and empirical correction by offwet forces. J Am Chem Soc 114:8191–8201
Mulliken RS (1955) Electronic population analysis on LCAO MO molecular wave functions. IV. Bonding and antibonding in LCAO and valence bond theories. J Chem Phys 23:2343–2346
Acknowledgments
Financial supports of this project by research council of Shiraz University of Medical Sciences are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miri, R., Razzaghi-asl, N. & Mohammadi, M.K. QM study and conformational analysis of an isatin Schiff base as a potential cytotoxic agent. J Mol Model 19, 727–735 (2013). https://doi.org/10.1007/s00894-012-1586-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1586-x