Abstract
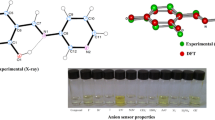
The relative binding affinities of several anions towards 2-nitroazophenol thiourea-based receptors were studied using density functional theory (DFT) in the gas phase and in chloroform solvent via PCM calculations. Both receptors have five distinctive NH and OH hydrogen donor atoms. All receptor–anion complexes are characterized by five intermolecular hydrogen bonds. The binding free energies are strongly influenced by a dielectric medium, and the solvation effect alters the trend of anion binding to the receptor. The calculated order of anion binding affinity for the receptor in chloroform, H2PO −4 > AcO− > F− > Cl− > HSO −4 > NO −3 , is in excellent accord with experimental findings. The overall order of binding affinity is attributed to the basicity of the anion, the effect of solvation, and the number of proton acceptors available. Calculations of the NMR and UV-vis spectra strongly support the experimental characterization of the receptor–anion complexes. Explicit solvent molecular dynamics simulations of selected receptor–anion complexes were also carried out. Analysis of the structural descriptors revealed that the anions were strongly bound within the binding pocket via hydrogen-bonding interactions to the five receptor protons throughout the simulation.

Chromogenic anion sensing of azophenol thiourea-based receptor.






Similar content being viewed by others
References
Sessler JL, Gale PA, Cho WS (2006) Anion receptor chemistry. Royal Society of Chemistry, Cambridge
Gunnlaugsson T, Glynn M, Tocci GM, Kruger PE, Pfeffer FM (2006) Coord Chem Rev 250:3094–3117
Gale PA, Quesada R (2006) Coord Chem Rev 250:3219–3244
Wong MW, Ghosh T, Maiya BG (2004) J Phys Chem A 108:11249–11259
Lee SJ, Jung JH, Seo J, Yoon I, Park KM, Lindoy LF, Lee SS (2006) Org Lett 8:1641–1643
Singh NJ, Jun EJ, Chellappan K, Thangadurai D, Chandran RP, Hwang IC, Yoon J, Kim KS (2007) Org Lett 9:485–488
Lu QS, Dong L, Zhang J, Li J, Jiang L, Huang Y, Qin S, Hu CW, Yu XQ (2009) Org Lett 11:669–672
Li AF, Wang JH, Wang F, Jiang YB (2010) Chem Soc Rev 39:3729–3745
Kato R, Nishizawa S, Hayashita T, Teramae N (2001) Tetrahedron Lett 42:5053–5056
Lee DH, Lee KH, Hong JI (2001) Org Lett 3:5–8
Lee DH, Lee HY, Lee KH, Hong JI (2001) Chem Commun 1188–1189
Lee DH, Im JH, Son SU, Chung YK, Hong JI (2003) J Am Chem Soc 125:7752–7753
Chen YJ, Chung WS (2009) Eur J Org Chem 4770–4776
Ruangpornvisuti VJ (2004) Mol Struct Theochem 686:47–55
Mondal CK, Lee JY (2006) J Theor Comput Chem 5:857–869
Jose DA, Singh A, Das A, Ganguly B (2007) Tetrahedron Lett 48:3695–3698
Rakrai W, Morakot N, Keawwangchai S, Kaewtong C, Wanno B, Ruangpornvisuti V (2011) Struct Chem 22:839–847
Xie H, Wong MW (2012) Aust J Chem 65:303–313
Becke DA (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Wong MW (1996) Chem Phys Lett 256:391–399
Cossi M, Barone V, Cammi R, Tomasi J (1996) Chem Phys Lett 255:327–335
Barone V, Cossi M, Tomasi J (1997) J Chem Phys 107:3210–3221
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Messerschmidt M, Wagner A, Wong MW, Luger P (2002) J Am Chem Soc 124:732–733
Wolinski K, Hinton JF, Pulay P (1990) J Am Chem Soc 112:8251–8260
Cheeseman JR, Trucks GW, Keith T, Frisch MJ (1996) J Phys Chem 104:5497–5509
Bauernschmitt R, Ahlrichs R (1996) Chem Phys Lett 256:454–464
Casida ME, Jamorski C, Casida KC, Salahub DR (1998) J Chem Phys 108:4439–4449
Jacquemin D, Adamo C (2012) Int J Quantum Chem 112:2135–2141
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03. Gaussian Inc., Wallingford
Case DA, Darden TA, Cheatham TE III, Simmerling CL, Wang J, Duke RE, Luo R, Merz KM, Pearlman DA, Crowley M, Walker RC, Zhang W, Wang B, Hayik S, Roitberg A, Seabra G, Wong KF, Paesani F, Wu X, Brozell S, Tsui V, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Beroza P, Mathews DH, Schafmeister C, Ross WS, Kollman PA (2006) AMBER 9. University of California, San Francisco
Pearlman DA, Case DA, Caldwell JW, Ross WR, Cheatham TE III, DeBolt S, Ferguson D, Seibel G, Kollman PA (1995) Comp Phys Commun 91:1–41
Cieplak P, Caldwell JW, Kollman PA (2001) J Comput Chem 22:1048–1057
Wavefunction Inc. (2010) SPARTAN 10. Wavefunction Inc., Irvine
Bondi A (1964) J Phys Chem 68:441
Desiraju GR (1991) Acc Chem Res 24:290–296
Ran J, Wong MW (2009) Aust J Chem 62:1062–1067
Wenthold PG, Squires RR (1985) J Phys Chem 99:2002–2005
Wiskur SL, Ait-Haddou H, Lavigne JJ, Anslyn EV (2001) Acc Chem Res 34:963–972
Böes ES, Andrade JA, Stassen H, Goncalves PFB (2007) Chem Phys Lett 436:362–367
Marques MAL, Ullrich CA, Nogueira F, Rubio A, Burke K, Gross EKU (eds) (2006) Time-dependent density functional theory. Springer, Berlin
Dreuw A, Weisman J, Head-Gordon M (2003) J Chem Phys 119:2943–2946
Acknowledgments
This research was supported by the National University of Singapore (grant no: R-143-000-253-112).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 148 kb)
Rights and permissions
About this article
Cite this article
Wong, M.W., Xie, H. & Kwa, S.T. Anion recognition by azophenol thiourea-based chromogenic sensors: a combined DFT and molecular dynamics investigation. J Mol Model 19, 205–213 (2013). https://doi.org/10.1007/s00894-012-1530-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1530-0