Abstract
Objective
The aim of this study was to evaluate the acidogenicity of dual-species biofilms of bifidobacteria and Streptococcus mutans.
Materials and methods
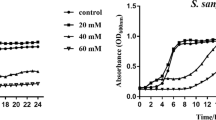
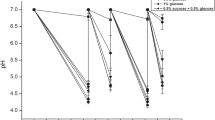
The following strains were tested: Bifidobacterium dentium DSM20436, Parascardovia denticolens DSM10105, and Scardovia inopinata DSM10107. Streptococcus mutans UA159 and Lactobacillus acidophilus ATCC4356 were used as control. Bifidobacteria were studied planktonically as they were not able to form monospecies biofilm, they were grown in biofilms associated with S. mutans. Endogenous polysaccharide reserves of cultures at log phase were depleted. Standardized suspensions of the microorganisms were incubated in growth media supplemented with 10 mM glucose, lactose, raffinose, glucose, or xylitol. S. mutans biofilms were grown on glass cover slips for 24 h to which bifidobacteria were added. After 24 h, the dual-species biofilms were exposed to the same carbon sources, and after 3 h, the pH of spent culture media and concentrations of organic acids were measured. Statistical analyses were carried out using ANOVA and Tukey’s test (α = 0.05).
Results
A higher pH drop was observed when S. mutans was associated with P. denticolens or S. inopinata, in either planktonic or biofilm cultures, than with S. mutans alone. Bifidobacteria showed a higher pH drop in the presence of raffinose than S. mutans or L. acidophilus.
Conclusions
Dual-species biofilms of bifidobacteria and S. mutans produced more acid and greater pH drops than biofilms of S. mutans alone.
Clinical relevance
New insights on the complex process of caries pathogenicity contribute to the establishment of preventive and therapeutic measures, in particular in specific cases, such as in early childhood caries.
Similar content being viewed by others
References
Bretz WA, Corby PM, Hart TC, Costa S, Coelho MQ, Weyant RJ, Robinson M, Schork NJ (2005) Dental caries and microbial acid production in twins. Caries Res 39:168–172
Marsh PD (1994) Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 8:263–271
Mantzourani M, Gilbert SC, Sulong HN, Sheehy EC, Tank S, Fenlon M, Beighton D (2009) The isolation of bifidobacteria from occlusal carious lesions in children and adults. Caries Res 43:308–313
Almståhl A, Lingström P, Eliasson L, Carlén A (2013) Fermentation of sugars and sugar alcohols by plaque Lactobacillus strains. Clin Oral Investig 17:1465–1470
Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ (2008) Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol 46:1407–1417
Modesto M, Biavati B, Mattarelli P (2006) Occurrence of the family Bifidobacteriaceae in human dental caries and plaque. Caries Res 40:271–276
Hojo K, Mizoguchi C, Taketomo N, Ohshima T, Gomi K, Arai T, Maeda N (2007) Distribution of salivary Lactobacillus and Bifidobacterium species in periodontal health and disease. Biosci Biotechnol Biochem 71:152–157
Beighton D, Gilbert SC, Clark D, Mantzourani M, Al-Haboubi M, Ali F, Ransome E, Hodson N, Fenlon M, Zoitopoulos L, Gallagher J (2008) Isolation and identification of Bifidobacteriaceae from human saliva. Appl Environ Microbiol 74:6457–6460
Kaur R, Gilbert SC, Sheehy EC, Beighton D (2013) Salivary levels of bifidobacteria in caries-free and caries-active children. Int J Paediatr Dent 23:32–38
Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, Papadopolou E, Dewhirst FE (2011) Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol 49:1464–1474
Brighenti FL, Luppens SB, Delbem AC, Deng DM, Hoogenkamp MA, Gaetti-Jardim E Jr, Dekker HL, Crielaard W, ten Cate JM (2008) Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 42:148–154
De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Bacteriol 23:130–135
McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P (2005) Development and characterization of a simple perfused oral microcosm. J Appl Microbiol 98:624–634
Exterkate RA, Crielaard W, ten Cate JM (2010) Different response to amine fluoride by Streptococcus mutans and polymicrobial biofilms in a novel high-throughput active attachment model. Caries Res 44:372–379
Silva TC, Pereira AFF, Exterkate RAM, Bagnato VS, Buzalaf MAR, Machado MAAM, ten Cate JM, Crielaard W, Deng DM (2012) Application of an active attachment model as a high-throughput demineralization biofilm model. J Dent 40:41–47
Davey AL, Rogers AH (1984) Multiple types of the bacterium Streptococcus mutans in the human mouth and their intra-family transmission. Arch Oral Biol 29:453–460
Kara D, Luppens SB, ten Cate JM (2006) Differences between single- and dual-species biofilms of Streptococcus mutans and Veillonella parvula in growth, acidogenicity and susceptibility to chlorhexidine. Eur J Oral Sci 114:58–63
Haukioja A, Söderling E, Tenovuo J (2008) Acid production from sugars and sugar alcohols by probiotic lactobacilli and bifidobacteria in vitro. Caries Res 42:449–453
Moynihan PJ, Ferrier S, Blomley S, Wright WG, Russell RR (1998) Acid production from lactulose by dental plaque bacteria. Lett Appl Microbiol 27:173–177
Nakajo K, Takahashi N, Beighton D (2011) Resistance to acidic environments of caries-associated bacteria: Bifidobacterium dentium and Bifidobacterium longum. Caries Res 44:431–437
Self Nutrition Data. http://nutritiondata.self.com. Accessed 05 May 2016
Fontana M, González-Cabezas C (2012) Are we ready for definitive clinical guidelines on xylitol/polyol use? Adv Dent Res 24:123–128
Twetman S (2012) Are we ready for caries prevention through bacteriotherapy? Braz Oral Res 26(Suppl 1):64–70
Crociani F, Biavati B, Alessandrini A, Chiarini C, Scardovi V (1996) Bifidobacterium inopinatum sp. nov. and Bifidobacterium denticolens sp. nov., two new species isolated from human dental caries. Int J Syst Bacteriol 46:564–571
Downes J, Mantzourani M, Beighton D, Hooper S, Wilson MJ, Nicholson A, Wade WG (2011) Scardovia wiggsiae sp. nov., isolated from the human oral cavity and clinical material, and emended descriptions of the genus Scardovia and Scardovia inopinata. Int J Syst Evol Microbiol 61:25–29
Henne K, Rheinberg A, Melzer-Krick B, Conrads G (2015) Aciduric microbial taxa including Scardovia wiggsiae and Bifidobacterium spp. in caries and caries free subjects. Anaerobe 35(Pt A):60–65
Acknowledgments
The authors thank Coordination for the Improvement of Higher Education Personnel– CAPES/Brazil (Grant #3755/10-0) and São Paulo Research Foundation – FAPESP/Brazil (Grant #10/02063-1), and the staff of the Laboratory of Oral Microbiology – Academic Centre for Dentistry Amsterdam (ACTA) – Vrije Universiteit Amsterdam – Netherlands for its contribution (laboratory facilities and consumables). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The work was supported by the Coordination for the Improvement of Higher Education Personnel – CAPES/Brazil (Grant #3755/10–0) and São Paulo Research Foundation – FAPESP/Brazil (Grant #2010/02,063–1).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards.
Electronic supplementary material
ESM 1
(DOCX 2286 kb)
Rights and permissions
About this article
Cite this article
de Matos, B.M., Brighenti, F.L., Do, T. et al. Acidogenicity of dual-species biofilms of bifidobacteria and Streptococcus mutans . Clin Oral Invest 21, 1769–1776 (2017). https://doi.org/10.1007/s00784-016-1958-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1958-1