Abstract
Objectives
The purpose of this study was to evaluate efficacy of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) and casein phosphopeptide-amorphous calcium fluoride phosphate (CPP-ACFP) containing pastes among individuals with Sjögren’s syndrome (SS).
Materials and methods
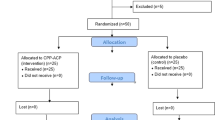
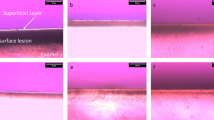
Thirty patients were randomised into three groups: CPP-ACP, CPP-ACFP, and 0.05 % NaF to be used two times a day during a 28-day experimental period. Saliva was analysed for flow rate, pH, buffering capacity and mineral concentrations. Dental plaque was examined for pH. Following the formation of artificial carious lesion, participants wore enamel slabs for an in situ remineralisation study. Remineralisation potential was examined using scanning electron microscope (SEM) and energy dispersive spectroscopic (EDS) technique. SE microphotographs were subsequently analysed for area, diameter, perimeter, roundness and the number of enamel defects and percentage of tooth surface affected by defects.
Results
At the end of the experimental period, a slight increase of salivary pH could have been observed. No differences in mineral composition of saliva were noted. The use of CPP-ACP and CPP-ACFP contributed to a significant rise of plaque pH. Image analysis revealed excessive reduction of defects’ dimensions in the three experimental groups, and a decrease of the number of enamel defects in the CPP-ACP and CPP-ACFP groups. The EDS analysis did not show differences in Ca/P, Ca/O and P/O ratios in any of the treatment groups.
Conclusion
CPP-ACP and CPP-ACFP hold promise as remineralising agents for patients with SS.
Clinical relevance
Pastes containing CPP-ACP/CPP-ACFP show enhanced remineralisation potential compared with NaF mouthrinse in patients with SS.






Similar content being viewed by others
References
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH (2002) Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61:554–558
Mavragani CP, Moutsopoulos NM, Moutsopoulos HM (2006) The management of Sjögren’s syndrome. Nat Clin Pract Rheumatol 2:252–261
Guggenheimer J, Moore PA (2003) Xerostomia: etiology, recognition and treatment. J Am Dent Assoc 134:61–69
Sreebny LM, Valdini A (1987) Xerostomia: a neglected symptom. Arch Intern Med 147:1333–1337
Abdelghany A, Nolan A, Freeman R (2011) Treating patients with dry mouth: general dental practitioners’ knowledge, attitudes and clinical management. Br Dent J 211:E21
Jansma J, Vissink A, Spijkervet FKL, Roodenburg JLN, Panders AK, Vermey A, Szabo BG, Gravenmade EJ (1992) Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiotherapy. Cancer 70:2171–2180
Soto-Rojas AE, Kraus K (2002) The oral side of Sjögren syndrome. Diagnosis and treatment. A review. Arch Med Res 33:95–106
Plemons JM, Al-Hashimi I, Marek CL, American Dental Association Council on Scientific Affairs (2014) Managing xerostomia and salivary gland hypofunction: executive summary of a report from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 145:867–873
Dreizen SA, Brown LR, Daly TE, Drane JB (1977) Prevention of xerostomia-related dental caries in irradiated cancer patient. J Dent Res 56:99–104
Horiot JC, Schraub S, Bone MC, Bain Y, Ramadier J, Chaplain G, Nabid N, Thevenot B, Bransfield D (1983) Dental preservation in patients irradiated for head and neck tumours: a 10-year experience with topical fluoride and a randomized trial between two fluoridation methods. Radiother Oncol 1:77–82
Meyerowitz C, Featherstone JDB, Billings RJ, Eisenberg AD, Fu J, Shariati M, Zero DT (1991) Use of an intra-oral model to evaluate 0.05 % sodium fluoride mouthrinse in radiation-induced hyposalivation. J Dent Res 70:894–898
Joyston-Bechal S, Hayes K, Davenport ES, Hardie JM (1992) Caries incidence, mutans streptococci and lactobacilli in irradiated patients during a 12-month preventive programme using chlorhexidine and fluoride. Caries Res 26:384–390
Reynolds EC (2009) Casein phosphopeptide-amorphous calcium phosphate: the scientific evidence. Adv Dent Res 21:25–29
Papas A, Russell D, Singh M, Stack K, Kent R, Triol C, Winston A (1999) Double blind trial of a remineralizing dentifrice in the prevention of caries in a radiation therapy population. Gerodontology 16:2–11
Teranaka T, Koulourides T (1987) Effect of a 100-ppm fluoride mouthrinse on experimental root caries in humans. Caries Res 21:326–332
Ferrazzano GF, Amato I, Cantile T, Sangianantoni G, Ingenito A (2011) In vivo remineralising effect of GC tooth mousse on early dental enamel lesions: SEM analysis. Int Dent J 61:210–216
Nongonierma AB, FitzGerald RJ (2012) Biofunctional properties of caseinophosphopeptides in the oral cavity. Caries Res 46:234–267
Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC (2001) Remineralization of enamel subsurface lesions by sugar-free gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res 80:2066–2070
Cai F, Shen P, Morgan MV, Reynolds EC (2003) Remineralization of enamel subsurface lesions in situ by sugar-free lozenges containing casein phosphopeptide-amorphous calcium phosphate. Aust Dent J 48:240–243
Reynolds EC, Cai F, Shen P, Walker GD (2003) Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J Dent Res 82:206–211
Walker G, Cai F, Shen P, Reynolds C, Ward B, Fone C, Honda S, Koganei M, Oda M, Reynolds E (2006) Increased remineralization of tooth enamel by milk containing added casein phosphopeptide-amorphous calcium phosphate. J Dairy Res 73:74–78
Manton DJ, Walker GD, Cai F, Cochrane NJ, Shen P, Reynolds EC (2008) Remineralization of enamel subsurface lesions in situ by the use of three commercially available sugar free gums. Int J Paediatr Dent 18:284–290
Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, Reynolds C (2008) Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res 87:344–348
Cai F, Shen P, Walker GD, Reynolds C, Yuan Y, Reynolds EC (2009) Remineralization of enamel subsurface lesions by chewing gum with added calcium. J Dent 37:763–768
Walker GD, Cai F, Shen P, Bailey D, Yuan Y, Cochrane NJ, Reynolds C, Reynolds EC (2009) Consumption of milk with added casein phosphopeptide-amorphous calcium phosphate remineralizes enamel subsurface lesions in situ. Aust Dent J 54:245–249
Walker GD, Cai F, Shen P, Adams GG, Reynolds C, Reynolds EC (2010) Casein phosphopeptide-amorphous calcium phosphate incorporated into sugar confections inhibits the progression of enamel subsurface lesions in situ. Caries Res 44:33–40
Shen P, Manton DJ, Cochrane NJ, Walker GD, Yuan Y, Reynolds C, Reynolds EC (2011) Effect of added calcium phosphate on enamel remineralization by fluoride in a randomized controlled in situ trial. J Dent 39:518–525
Cochrane NJ, Shen P, Byrne SJ, Walker GD, Adams GG, Yuan Y, Reynolds C, Hoffmann B, Dashper SG, Reynolds EC (2012) Remineralisation by chewing sugar-free gums in a randomised, controlled in situ trial including dietary intake and gauze to promote plaque formation. Caries Res 46:147–155
Thepyou R, Chanmitkul W, Thanatvarakom O, Hamba H, Chob-Isara W, Trairatvorakul C, Tagami J (2013) Casein phosphopeptide-amorphous calcium phosphate and glass ionomer show distinct effects in the remineralization of proximal artificial caries lesion in situ. Dent Mater J 32:648–653
Yengopal V, Mickenautsch S (2009) Caries preventive effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP): a meta-analysis. Acta Odontol Scand 67:321–332
Gupta R, Prakash V (2011) CPP-ACP complex as a new adjunctive agent for remineralisation: a review. Oral Health Prev Dent 9:151–165
Li J, Xie X, Wasng Y, Yin W, Antoun JS, Farella M, Mei L (2014) Long-term remineralizing effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on early caries lesions in vivo: a systematic review. J Dent 42:769–777
Smith RN, Elcock C, Abdellatif A, Bäckman B, Russell JM, Brook AH (2009) Enamel defects in extracted and exfoliated teeth from patients with Amelogenesis Imperfecta, measured using the extended enamel defects index and image analysis. Arch Oral Biol 54(Suppl 1):S86–S92
Peric TO, Markovic DL, Radojevic VJ, Jancic Heinemann RM, Petrovic BB, Lamovec JS (2014) Influence of pastes containing casein phosphopeptide-amorphous calcium phosphate on surface of demineralised enamel. J Appl Biomater Funct Mater 12:234–239
Kumar VLN, Itthagarun A, King NM (2008) The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: an in vitro study. Aust Dent J 53:34–40
Ogata K, Warita S, Shimazu K, Kawakami T, Aoyagi K, Karibe H (2010) Combined effect of paste containing casein phosphopeptide-amorphous calcium phosphate and fluoride on enamel lesions: an in vitro pH-cycling study. Pediatr Dent 32:433–438
Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds EC (2008) Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res 42:88–97
Reynolds EC (1997) Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res 76:1587–1595
Goldstein J, Newbury DE, Joy DC, Lyman CE, Echlin P, Lifshin E, Sawyer L, Michael JR (2003) Scanning electron microscopy and x-ray microanalysis, 3rd edn. Springer, New York
Little MF, Posen J, Singer L (1962) Chemical and physical properties of altered and sound enamel. 3. Fluoride and sodium content. J Dent Res 41:784–789
Hannig C, Gaeding A, Basche S, Richter G, Helbig R, Hannig M (2013) Effect of conventional mouthrinses on initial bioadhesion to enamel and dentin in situ. Caries Res 47:150–161
Aps JKM, Martens LC (2005) Review: the physiology of saliva and transfer of drugs into saliva. Forensic Sci Int 150:119–131
Chiappin S, Antonelli G, Gatti R, De Palo EF (2007) Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta 383:30–40
Bardow A, Nyvad B, Nauntofte B (2001) Relationships between medication intake, complaints of dry mouth, salivary flow rate and composition, and the rate of tooth demineralization in situ. Arch Oral Biol 46:413–423
Ericson D, Bratthall D (1989) Simplified method to estimate salivary buffer capacity. Scand J Dent Res 97:405–407
Cheaib Z, Ganss C, Lamanda A, Turgut MD, Lussi A (2012) Comparison of three strip-type tests and two laboratory methods for salivary buffering analysis. Odontology 100:67–75
McMaugh DR (1977) A comparative analysis of the colour matching ability of dentists, dental students, and ceramic technicians. Aust Dent J 22:165–167
Kitasako Y, Burrow MF, Stacey M, Huq L, Reynolds EC, Tagami J (2008) Comparative analysis of three commercial saliva testing kits with a standard saliva buffering test. Aust Dent J 53:140–144
Ericsson Y (1959) Clinical investigations of the salivary buffering action. Acta Odontol Scand 17:131–165
Anderson P, Hector MP, Rampersad MA (2001) Critical pH in resting and stimulated whole saliva in groups of children and adults. Int J Paediatr Dent 11:266–273
Kalk WWI, Vissink A, Spijkervet FKL, Bootsma H, Kallenberg CGM, Nieuw Amerongen AV (2001) Sialometry and sialochemistry: diagnostic tools for Sjögren’s syndrome. Ann Rheum Dis 60:1110–1116
Kalk WW, Vissink A, Stegenga B, Bootsma H, Nieuw Amerongen AV, Kallenberg CG (2002) Sialometry and sialochemistry: a non-invasive approach for diagnosing Sjogren’s syndrome. Ann Rheum Dis 61:137–144
Gao XJ, Elliott JC, Anderson P (1991) Scanning and contact microradiographic study of the effect of degree of saturation on the rate of enamel demineralization. J Dent Res 70:1332–1337
Rose RK (2000) Binding characteristics of streptococcus mutans for calcium and casein phosphopeptide. Caries Res 34:427–431
Rose RK, Turner SJ (1998) Fluoride-induced enhancement of diffusion in streptococcal model plaque biofilms. Caries Res 32:227–232
Rose RK (2000) Effects of an anticariogenic casein phosphopeptide on calcium diffusion in streptococcal model dental plaques. Arch Oral Biol 45:569–575
Caruana PC, Mulaify SA, Moazzez R, Bartlett D (2009) The effect of casein and calcium containing paste on plaque pH following a subsequent carbohydrate challenge. J Dent 37:522–526
Sissons CH, Wong L, Shu M (1998) Factors affecting the resting pH of in vitro human microcosm dental plaque and Streptococcus mutans biofilms. Arch Oral Biol 43:93–102
Walsh LJ (2006) Dental plaque fermentation and its role in caries risk assessment. Int Dent SA 8:34–40
Chaussain C, Opsahl Vital S, Viallon V, Vermelin L, Haignere C, Sixou M, Lasfargues JJ (2010) Interest in a new test for caries risk in adolescents undergoing orthodontic treatment. Clin Oral Investig 14:177–185
Schüpbach P, Neeser JR, Golliard M, Rouvet M, Guggenheim B (1996) Incorporation of caseinoglycomacropeptide and caseinphosphopeptide into the salivary pellicle inhibits adherence of mutans streptococci. J Dent Res 75:1779–1788
Rahiotis C, Vougiouklakis G, Eliades G (2008) Characterization of oral films formed in the presence of a CPP-ACP agent: an in situ study. J Dent 36:272–280
Conflict of interests
The authors declare that they have no competing interests. GC Int. (Tokyo, Japan) and Curaden International AG (Kriens, Switzerland) provided the materials, but no funding for this study, and did not have role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peric, T., Markovic, D., Petrovic, B. et al. Efficacy of pastes containing CPP-ACP and CPP-ACFP in patients with Sjögren’s syndrome. Clin Oral Invest 19, 2153–2165 (2015). https://doi.org/10.1007/s00784-015-1444-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1444-1