Abstract
Objective
This study aimed to check the effect of zoledronic acid (ZA) at subtoxic dose on human osteoblasts (HOs) in terms of cell viability, apoptosis occurrence, and differentiation induction. ZA belongs to the family of bisphosphonates (BPs), largely used in the clinical practice for the treatment of bone diseases, often associated with jaw osteonecrosis onset. Their pharmacological action consists in the direct block of the osteoclast-mediated bone resorption along with indirect action on osteoblasts.
Materials and methods
HOs were treated choosing the highest limit concentration (10−5 M) which does not induce toxic effects. Live/dead staining, flow cytometry, mitochondrial membrane potential assay, osteocalcin western blotting, gp38 RT-PCR, collagen type I, PGE2, and IL-6 ELISA assays were performed.
Results
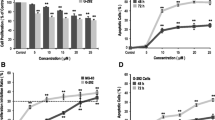
Similar viability level between control and ZA-treated samples is found along with no significant increase of apoptotic and necrotic cells in ZA-treated sample. To establish if an early apoptotic pathway was triggered, Bax expression and mitochondrial membrane potential were evaluated finding a higher protein expression in control sample and a good integrity of mitochondrial membrane in both experimental points. Type I collagen secretion and alkaline phosphatase (ALP) activity appear increased in ZA-treated sample, osteocalcin expression level is reduced in ZA-treated cells, whereas no modifications of gp38 mRNA level are evidenced. No statistical differences are identified in PGE2 secretion level whereas IL-6 secretion is lower in ZA-treated HOs with respect to control ones.
Conclusions
These results highlight that ZA, delaying the osteoblastic differentiation process versus the osteocytic lineage, strengthens its pharmacological activity enhancing bone density.
Clinical relevance
The knowledge of ZA effects on osteoblasts at subtoxic dose allows to improve therapeutic protocols in order to strengthen drug pharmacological activity through a combined action on both osteoclastic and osteoblastic cells.










Similar content being viewed by others
References
Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:65–76
Rauch F, Travers R, Plotkin H, Glorieux FH (2002) The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfect. J Clin Invest 110:1293–1299
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Zheng M (2004) Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 96:879–882
Santini D, Vincenzi B, Hannon RA, Brown JE, Dicuonzo G, Angeletti S, La Cesa A, Coleman RE, Tonini G, Budillon A, Caraglia M, Holen I (2006) Changes in bone resorption and vascular endothelial growth factor after a single zoledronic acid infusion in cancer patients with bone metastases from solid tumours. Oncol Rep 15:1351–1357
Green LR (2002) Bisphosphonates in cancer therapy. Curr Opin Oncol 14:609–615
Rogers MJ (2003) New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des 9:2643–2658
Fleisch H (2002) Development of bisphosphonates. Breast Cancer Res 4(1):30–34
Mashiba T, Hirano T, Turner CH, Forwood MH, Johnston CC, Burr DB (2000) Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 15:613–620
Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC (2000) Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res 60:6001–6007
Bagan JV, Jimenez Y, Murillo J, Hernandez S, Poveda R, Sanchis JM, Diaz JM, Scully C (2006) Jaw osteonecrosis associated with bisphosphonates: multiple exposed areas and its relationship to teeth extractions. Study of 20 cases. Oral Oncol 42:327–329
Rizos EC, Milionis HJ, Elisaf MS (2006) Fever with rash following zoledronic acid administration. Clin Exp Rheumatol 24:455
Reid IR, Bolland MJ, Grey AB (2007) Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone 41:318–320
Vitté C, Fleisch H, Guenther HL (1996) Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast-mediated resorption. Endocrinology 137(6):2324–2333
Viereck V, Emons G, Lauck V, Blaschke S, Gründker C, Hofbauer LC (2002) Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun 291:680–686
Lee YJ, Jeong JK, Seol JW, Xue M, Jackson C, Park SY (2013) Activated protein C differentially regulates both viability and differentiation of osteoblasts mediated by bisphosphonates. Exp Mol Med 45:e9
Tsuchimoto M, Azuma Y, Higuchi SI, Hirata N, Kiyoki M, Yamamoto I (1994) Alendronate modulates osteogenesis of human osteoblastic cells in vitro. Jpn J Pharmacol 66:25–33
Abe Y, Kawakami A, Nakashima T, Ejima E, Fujiyama K, Kiriyama T, Ide A, Sera N, Usa T, Tominaga T, Ashizawa K, Yokoyama N, Eguchi K (2000) Etidronate inhibits human osteoblast apoptosis by inhibition of pro-apoptotic factor(s) produced by activated T cells. J Lab Clin Med 136:344–354
Pan B, To LB, Farrugia AN, Findlay DM, Green J, Gronthos S, Evdokiou A, Lynch K, Atkins GJ, Zannettino AC (2004) The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralisation of human bone-derived cells in vitro. Bone 34:112–123
Xiong Y, Yang HJ, Feng J, Shi ZL, Wu LD (2009) Effects of alendronate on the proliferation and osteogenic differentiation of MG-63 cells. J Int Med Res 37:407–416
Simon MJ, Niehoff P, Kimmig B, Wiltfang J, Açil Y (2010) Expression profile and synthesis of different collagen types I, II, III, and V of human gingival fibroblasts, osteoblasts, and SaOS-2 cells after bisphosphonate treatment. Clin Oral Investig 14:51–58
Naidu A, Dechow PC, Spears R, Wright JM, Kessler HP, Opperman LA (2008) The effects of bisphosphonates on osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:5–13
Kim HK, Kim JH, Abbas AA, Yoon TR (2009) Alendronate enhances osteogenic differentiation of bone marrow stromal cells: a preliminary study. Clin Orthop Relat Res 467:3121–3128
Orriss IR, Key ML, Colston KW, Arnett TR (2009) Inhibition of osteoblast function in vitro by aminobisphosphonates. J Cell Biochem 106:109–118
Pozzi S, Vallet S, Mukherjee S, Cirstea D, Vaghela N, Santo L, Rosen E, Ikeda H, Okawa Y, Kiziltepe T, Schoonmaker J, Xie W, Hideshima T, Weller E, Bouxsein ML, Munshi NC, Anderson KC, Raje N (2009) High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin Cancer Res 15:5829–5839
Bellido T, Plotkin LI (2011) Novel actions of bisphosphonates in bone: preservation of osteoblast and osteocyte viability. Bone 49:50–55
Igarashi K, Hirafuji M, Adachi H, Shinoda H, Mitani H (1997) Effects of bisphosphonates on alkaline phosphatase activity, mineralization, and prostaglandin E2 synthesis in the clonal osteoblast-like cell line MC3T3-E1. Prostaglandins Leukot Essent Fat Acids 56:121–125
Bellido T, Borba VZ, Roberson P, Manolagas SC (1997) Activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology 138:3666–3676
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Schmidt C, Steinbach G, Decking R, Claes LE, Ignatius AA (2003) IL-6 and PGE2 release by human osteoblasts on implant materials. Biomaterials 24:4191–4196
Murakami H, Takahashi N, Sasaki T, Udagawa N, Tanaka S, Nakamura I, Zhang D, Barbier A, Suda T (1995) A possible mechanism of the specific action of bisphosphonates on osteoclasts: tiludronate preferentially affects polarized osteoclasts having ruffled borders. Bone 17:137–144
Sinha RK, Morris F, Shah SA, Tuan RS (1994) Surface composition of orthopaedic implant metals regulates cell attachment, spreading, and cytoskeletal organization of primary human osteoblasts in vitro. Clin Orthop Relat Res 305:258–272
Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS (2004) Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 25:4105–4115
Greiner S, Kadow-Romacker A, Lübberstedt M, Schmidmaier G, Wildemann B (2007) The effect of zoledronic acid incorporated in a poly(D, L-lactide) implant coating on osteoblasts in vitro. J Biomed Mater Res A 80:769–775
Chan SL, Yu VC (2004) Proteins of the bcl-2 family in apoptosis signalling: from mechanistic insights to therapeutic opportunities. Clin Exp Pharmacol Physiol 31:119–128
Er E, Oliver L, Cartron PF, Juin P, Manon S, Vallette FM (2006) Mitochondria as the target of the pro-apoptotic protein Bax. Biochim Biophys Acta 1757:1301–1311
Zhang W, Pantschenko AG, McCarthy MB, Gronowicz G (2007) Bone-targeted verexpression of Bcl-2 increases osteoblast adhesion and differentiation and inhibits mineralization in vitro. Calcif Tissue Int 80:111–122
Lynch MP, Capparelli C, Stein JL, Stein GS, Lian JB (1998) Apoptosis during bone-like tissue development in vitro. J Cell Biochem 68:31–49
Marchisio M, Di Carmine M, Pagone R, Piattelli A, Miscia S (2005) Implant surface roughness influences osteoclast proliferation and differentiation. J Biomed Mater Res B Appl Biomater 75:251–256
Aubin JE, Turksen K (1996) Monoclonal antibodies as tools for studying the osteoblast lineage. Microsc Res Tech 33:128–140
Koch FP, Yekta SS, Merkel C, Ziebart T, Smeets R (2010) The impact of bisphosphonates on the osteoblast proliferation and collagen gene expression in vitro. Head Face Med 6:12
Neve A, Corrado A, Cantatore FP (2013) Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol 228:1149–1153
Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, Bonewald LF (2006) E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol 26:4539–4552
Moreau MF, Guillet C, Massin P, Chevalier S, Gascan H, Baslé MF, Chappard D (2007) Comparative effects of five bisphosphonates on apoptosis of macrophage cells in vitro. Biochem Pharmacol 73:718–723
Acknowledgments
The authors would like to thank Dott.ssa Piera Sozio for drug purity determinations, Dott.ssa Alessandra di Paolo for the kind gift of Zometa, and MIUR FIRB-Accordi di Programma 2010 Project “Processi degenerativi dei tessuti mineralizzati del cavo orale, impiego di biomateriali e controllo delle interazioni con i microrganismi dell’ambiente” for the fellowships attributed to Dr. V.L Zizzari and Dr. M. De Colli.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zara, S., De Colli, M., di Giacomo, V. et al. Zoledronic acid at subtoxic dose extends osteoblastic stage span of primary human osteoblasts. Clin Oral Invest 19, 601–611 (2015). https://doi.org/10.1007/s00784-014-1280-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1280-8