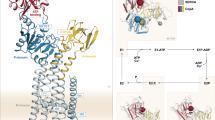
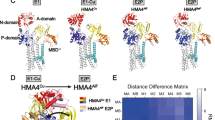
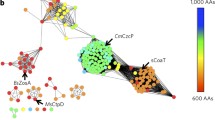
Abstract
Copper-transporting ATPases (Cu-ATPases) ATP7A and ATP7B play an essential role in human physiological function. Their primary function is to deliver copper to the secretory pathway and export excess copper from the cell for removal or further utilization. Cells employ Cu-ATPases in numerous physiological processes that include the biosynthesis of copper-dependent enzymes, lactation, and response to hypoxia. Biochemical studies of human Cu-ATPases and their orthologs have demonstrated that Cu-ATPases share many common structural and mechanistic characteristics with other members of the P-type ATPase family. Nevertheless, the Cu-ATPases have a unique coordinate environment for their ligands, copper and ATP, and additional domains that are required for sophisticated regulation of their intracellular localization and activity. Here, we review recent progress that has been made in understanding the structure of Cu-ATPases from the analysis of their individual domains and orthologs from microorganisms, and speculate about the implications of these findings for the function and regulation of human copper pumps.









Similar content being viewed by others
References
Mercer JF, Llanos RM (2003) Molecular and cellular aspects of copper transport in developing mammals. J Nutr 133:1481S–1484S
Veldhuis NA, Gaeth AP, Pearson RB, Gabriel K, Camakaris J (2009) The multi-layered regulation of copper translocating P-type ATPases. Biometals 22:177–190
Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY (2007) Function and regulation of human copper-transporting ATPases. Physiol Rev 87:1011–1046
Yuan DS, Dancis A, Klausner RD (1997) Restriction of copper export in Saccharomyces cerevisiae to a late Golgi or post-Golgi compartment in the secretory pathway. J Biol Chem 272:25787–25793
Mandal AK, Cheung WD, Arguello JM (2002) Characterization of a thermophilic P-type Ag+/Cu+-ATPase from the extremophile Archaeoglobus fulgidus. J Biol Chem 277:7201–7208
Gaballa A, Helmann JD (2003) Bacillus subtilis CPx-type ATPases: characterization of Cd, Zn, Co and Cu efflux systems. Biometals 16:497–505
Hatori Y, Lewis D, Toyoshima C, Inesi G (2009) Reaction cycle of Thermotoga maritima copper ATPase and conformational characterization of catalytically deficient mutants. Biochemistry 48:4871–4880
Chintalapati S, Al Kurdi R, van Scheltinga AC, Kuhlbrandt W (2008) Membrane structure of CtrA3, a copper-transporting P-type-ATPase from Aquifex aeolicus. J Mol Biol 378:581–595
Lewinson O, Lee AT, Rees DCA (2009) P-type ATPase importer that discriminates between essential and toxic transition metals. Proc Natl Acad Sci USA 106:4677–4682
Odermatt A, Suter H, Krapf R, Solioz M (1993) Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J Biol Chem 268:12775–12779
Mendelsohn BA et al (2006) Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab 4:155–162
Fan B, Rosen BP (2002) Biochemical characterization of CopA, the Escherichia coli Cu(I)-translocating P-type ATPase. J Biol Chem 277:46987–46992
Petris MJ et al (1996) Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J 15:6084–6095
Suzuki M, Gitlin JD (1999) Intracellular localization of the Menkes and Wilson’s disease proteins and their role in intracellular copper transport. Pediatr Int 41:436–442
Guo Y, Nyasae L, Braiterman LT, Hubbard AL (2005) NH2-terminal signals in ATP7B Cu-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol Gastrointest Liver Physiol 289:G904–G916
La Fontaine S, Mercer JF (2007) Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys 463:149–167
Abdel-Ghany SE, Muller-Moule P, Niyogi KK, Pilon M, Shikanai T (2005) Two P-type ATPases are required for copper delivery in Arabidopsis thaliana chloroplasts. Plant Cell 17:1233–1251
Walker JM, Tsivkovskii R, Lutsenko S (2002) Metallochaperone Atox1 transfers copper to the NH2-terminal domain of the Wilson’s disease protein and regulates its catalytic activity. J Biol Chem 277:27953–27959
Gonzalez-Guerrero M, Arguello JM (2008) Mechanism of Cu+-transporting ATPases: soluble Cu+ chaperones directly transfer Cu+ to transmembrane transport sites. Proc Natl Acad Sci USA 105:5992–5997
Pilankatta R, Lewis D, Adam CM, Inesi G (2009) High yield heterologous expression of WT and mutant Cu+ ATPase (ATP7B, Wilson disease protein) for functional characterization of catalytic activity and serine residues undergoing copper dependent phosphorylation. J Biol Chem 284:21307–21316
Arguello JM, Mandal AK, Mana-Capelli S (2003) Heavy metal transport CPx-ATPases from the thermophile Archaeoglobus fulgidus. Ann N Y Acad Sci 986:212–218
Rice WJ, Kovalishin A, Stokes DL (2006) Role of metal-binding domains of the copper pump from Archaeoglobus fulgidus. Biochem Biophys Res Commun 348:124–131
Hatori Y et al (2008) Intermediate phosphorylation reactions in the mechanism of ATP utilization by the copper ATPase (CopA) of Thermotoga maritima. J Biol Chem 283:22541–22549
Hensyl WR (ed) (2000) Bergey's manual of determinative bacteriology, 9th edn. Lippincott Williams and Wilkins, Philadelphia, p 304
Hung YH, Layton MJ, Voskoboinik I, Mercer JF, Camakaris J (2007) Purification and membrane reconstitution of catalytically active Menkes copper-transporting P-type ATPase (MNK; ATP7A). Biochem J 401:569–579
Vanderwerf SM, Cooper MJ, Stetsenko IV, Lutsenko S (2001) Copper specifically regulates intracellular phosphorylation of the Wilson’s disease protein, a human copper-transporting ATPase. J Biol Chem 276:36289–36294
Voskoboinik I et al (2003) Protein kinase-dependent phosphorylation of the Menkes copper P-type ATPase. Biochem Biophys Res Commun 303:337–342
Bartee MY, Ralle M, Lutsenko S (2009) The loop connecting metal-binding domains 3 and 4 of ATP7B is a target of a kinase-mediated phosphorylation. Biochemistry 48:5573–5581
Sazinsky MH et al (2007) Characterization and structure of a Zn2+ and [2Fe–2S]-containing copper chaperone from Archaeoglobus fulgidus. J Biol Chem 282:25950–25959
Rosen BP (2002) Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp Biochem Physiol A Mol Integr Physiol 133:689–693
Stokes DL, Auer M, Zhang P, Kuhlbrandt W (1999) Comparison of H+-ATPase and Ca2+-ATPase suggests that a large conformational change initiates P-type ion pump reaction cycles. Curr Biol 9:672–679
Yang Y, Mandal AK, Bredeston LM, Gonzalez-Flecha FL, Arguello JM (2007) Activation of Archaeoglobus fulgidus Cu(+)-ATPase CopA by cysteine. Biochim Biophys Acta 1768:495–501
Safaei R, Otani S, Larson BJ, Rasmussen ML, Howell SB (2008) Transport of cisplatin by the copper efflux transporter ATP7B. Mol Pharmacol 73:461–468
Odermatt A, Solioz M (1995) Two trans-acting metalloregulatory proteins controlling expression of the copper-ATPases of Enterococcus hirae. J Biol Chem 270:4349–4354
Wu CC, Rice WJ, Stokes DL (2008) Structure of a copper pump suggests a regulatory role for its metal-binding domain. Structure 16:976–985
Tsuda T, Toyoshima C (2009) Nucleotide recognition by CopA, a Cu+-transporting P-type ATPase. EMBO J 28:1782–1791
Mandal AK, Yang Y, Kertesz TM, Arguello JM (2004) Identification of the transmembrane metal binding site in Cu+-transporting PIB-type ATPases. J Biol Chem 279:54802–54807
Kuhlbrandt W (2004) Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol 5:282–295
Hatori Y, Majima E, Tsuda T, Toyoshima C (2007) Domain organization and movements in heavy metal ion pumps: papain digestion of CopA, a Cu+-transporting ATPase. J Biol Chem 282:25213–25221
Lubben M et al (2009) Structural model of the CopA copper ATPase of Enterococcus hirae based on chemical cross-linking. Biometals 22:363–375
Moller JV, Juul B, le Maire M (1996) Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim Biophys Acta 1286:1–51
Petris MJ et al (2002) Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J Biol Chem 277:46736–46742
Cater MA, La Fontaine S, Mercer JF (2007) Copper binding to the N-terminal metal-binding sites or the CPC motif is not essential for copper-induced trafficking of the human Wilson protein (ATP7B). Biochem J 401:143–153
Koonin EV, Tatusov RL (1994) Computer analysis of bacterial haloacid dehalogenases defines a large superfamily of hydrolases with diverse specificity. Application of an iterative approach to database search. J Mol Biol 244:125–132
Dmitriev O et al (2006) Solution structure of the N-domain of Wilson disease protein: distinct nucleotide-binding environment and effects of disease mutations. Proc Natl Acad Sci USA 103:5302–5307
Morgan CT, Tsivkovskii R, Kosinsky YA, Efremov RG, Lutsenko S (2004) The distinct functional properties of the nucleotide-binding domain of ATP7B, the human copper-transporting ATPase: analysis of the Wilson disease mutations E1064A, H1069Q, R1151H, and C1104F. J Biol Chem 279:36363–36371
Tsivkovskii R, Efremov RG, Lutsenko S (2003) The role of the invariant His-1069 in folding and function of the Wilson’s disease protein, the human copper-transporting ATPase ATP7B. J Biol Chem 278:13302–13308
Anthonisen AN, Clausen JD, Andersen JP (2006) Mutational analysis of the conserved TGES loop of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem 281:31572–31582
Toyoshima C, Norimatsu Y, Iwasawa S, Tsuda T, Ogawa H (2007) How processing of aspartylphosphate is coupled to lumenal gating of the ion pathway in the calcium pump. Proc Natl Acad Sci USA 104:19831–19836
Sorensen TL et al (2004) Localization of a K+-binding site involved in dephosphorylation of the sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem 279:46355–46358
DiDonato M, Narindrasorasak S, Forbes JR, Cox DW, Sarkar B (1997) Expression, purification, and metal binding properties of the N-terminal domain from the wilson disease putative copper-transporting ATPase (ATP7B). J Biol Chem 272:33279–33282
Lutsenko S, Petrukhin K, Cooper MJ, Gilliam CT, Kaplan JH (1997) N-terminal domains of human copper-transporting adenosine triphosphatases (the Wilson’s and Menkes disease proteins) bind copper selectively in vivo and in vitro with stoichiometry of one copper per metal-binding repeat. J Biol Chem 272:18939–18944
Banci L, Bertini I, Cantini F, Rosenzweig AC, Yatsunyk LA (2008) Metal binding domains 3 and 4 of the Wilson disease protein: solution structure and interaction with the copper(I) chaperone HAH1. Biochemistry 47:7423–7429
Achila D et al (2006) Structure of human Wilson protein domains 5 and 6 and their interplay with domain 4 and the copper chaperone HAH1 in copper uptake. Proc Natl Acad Sci USA 103:5729–5734
Jones CE, Daly NL, Cobine PA, Craik DJ, Dameron CT (2003) Structure and metal binding studies of the second copper binding domain of the Menkes ATPase. J Struct Biol 143:209–218
Walker JM et al (2004) The N-terminal metal-binding site 2 of the Wilson’s Disease Protein plays a key role in the transfer of copper from Atox1. J Biol Chem 279:15376–15384
Banci L et al (2005) An atomic-level investigation of the disease-causing A629P mutant of the Menkes protein, ATP7A. J Mol Biol 352:409–417
DeSilva TM, Veglia G, Opella SJ (2005) Solution structures of the reduced and Cu(I) bound forms of the first metal binding sequence of ATP7A associated with Menkes disease. Proteins 61:1038–1049
Banci L, Bertini I, Del Conte R, D’Onofrio M, Rosato A (2004) Solution structure and backbone dynamics of the Cu(I) and apo forms of the second metal-binding domain of the Menkes protein ATP7A. Biochemistry 43:3396–3403
Gitschier J, Moffat B, Reilly D, Wood WI, Fairbrother WJ (1998) Solution structure of the fourth metal-binding domain from the Menkes copper-transporting ATPase. Nat Struct Biol 5:47–54
Tsay MJ, Fatemi N, Narindrasorasak S, Forbes JR, Sarkar B (2004) Identification of the “missing domain” of the rat copper-transporting ATPase, atp7b: insight into the structural and metal binding characteristics of its N-terminal copper-binding domain. Biochim Biophys Acta 1688:78–85
Cater MA, Forbes J, La Fontaine S, Cox D, Mercer JF (2004) Intracellular trafficking of the human Wilson protein: the role of the six N-terminal metal-binding sites. Biochem J 380:805–813
Mercer JF, Barnes N, Stevenson J, Strausak D, Llanos RM (2003) Copper-induced trafficking of the cU-ATPases: a key mechanism for copper homeostasis. Biometals 16:175–184
Strausak D et al (1999) The role of GMXCXXC metal binding sites in the copper-induced redistribution of the Menkes protein. J Biol Chem 274:11170–11177
Banci L et al (2009) An NMR study of the interaction of the N-terminal cytoplasmic tail of the Wilson disease protein with copper(I)-HAH1. J Biol Chem 284:9354–9360
Yatsunyk LA, Rosenzweig AC (2007) Cu(I) binding and transfer by the N terminus of the Wilson disease protein. J Biol Chem 282:8622–8631
Ralle M, Lutsenko S, Blackburn NJ (2004) Copper transfer to the N-terminal domain of the Wilson disease protein (ATP7B): X-ray absorption spectroscopy of reconstituted and chaperone-loaded metal binding domains and their interaction with exogenous ligands. J Inorg Biochem 98:765–774
Arnesano F et al (2002) Metallochaperones and metal-transporting ATPases: a comparative analysis of sequences and structures. Genome Res 12:255–271
O’Halloran TV, Culotta VC (2000) Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem 275:25057–25060
Banci L, Bertini I, Del Conte R, Markey J, Ruiz-Duenas FJ (2001) Copper trafficking: the solution structure of Bacillus subtilis CopZ. Biochemistry 40:15660–15668
Hamza I, Schaefer M, Klomp LW, Gitlin JD (1999) Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis. Proc Natl Acad Sci USA 96:13363–13368
Huffman DL, O’Halloran TV (2001) Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem 70:677–701
Puig S et al (2007) Higher plants possess two different types of ATX1-like copper chaperones. Biochem Biophys Res Commun 354:385–390
Andres-Colas N et al (2006) The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J 45:225–236
Wernimont AK, Huffman DL, Lamb AL, O’Halloran TV, Rosenzweig AC (2000) Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat Struct Biol 7:766–771
Ralle M, Lutsenko S, Blackburn NJ (2003) X-ray absorption spectroscopy of the copper chaperone HAH1 reveals a linear two-coordinate Cu(I) center capable of adduct formation with exogenous thiols and phosphines. J Biol Chem 278:23163–23170
Pufahl RA et al (1997) Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science 278:853–856
Banci L, Bertini I, Chasapis CT, Rosato A, Tenori L (2007) Interaction of the two soluble metal-binding domains of yeast Ccc2 with copper(I)-Atx1. Biochem Biophys Res Commun 364:645–649
Banci L, Bertini I, Ciofi-Baffoni S, Gonnelli L, Su XC (2003) Structural basis for the function of the N-terminal domain of the ATPase CopA from Bacillus subtilis. J Biol Chem 278:50506–50513
Larin D et al (1999) Characterization of the interaction between the Wilson and Menkes disease proteins and the cytoplasmic copper chaperone, HAH1p. J Biol Chem 274:28497–28504
Banci L et al (2007) The different intermolecular interactions of the soluble copper-binding domains of the Menkes protein, ATP7A. J Biol Chem 282:23140–23146
Bunce J, Achila D, Hetrick E, Lesley L, Huffman DL (2006) Copper transfer studies between the N-terminal copper binding domains one and four of human Wilson protein. Biochim Biophys Acta 1760:907–912
Rosenzweig AC et al (1999) Crystal structure of the Atx1 metallochaperone protein at 1.02 A resolution. Structure 7:605–617
Wernimont AK, Yatsunyk LA, Rosenzweig AC (2004) Binding of copper(I) by the Wilson disease protein and its copper chaperone. J Biol Chem 279:12269–12276
Banci L et al (2009) Copper(I)-mediated protein–protein interactions result from suboptimal interaction surfaces. Biochem J 422:37–42
Op’t Holt BT, Merz KM Jr (2007) Insights into Cu(I) exchange in HAH1 using quantum mechanical and molecular simulations. Biochemistry 46:8816–8826
Banci L et al (2006) The Atx1–Ccc2 complex is a metal-mediated protein–protein interaction. Nat Chem Biol 2:367–368
Singleton C et al (2008) Structure and Cu(I)-binding properties of the N-terminal soluble domains of Bacillus subtilis CopA. Biochem J 411:571–579
Gonzalez-Guerrero M, Eren E, Rawat S, Stemmler TL, Arguello JM (2008) Structure of the two transmembrane Cu+ transport sites of the Cu+-ATPases. J Biol Chem 283:29753–29759
Mandal AK, Arguello JM (2003) Functional roles of metal binding domains of the Archaeoglobus fulgidus Cu(+)-ATPase CopA. Biochemistry 42:11040–11047
Fan B, Grass G, Rensing C, Rosen BP (2001) Escherichia coli CopA N-terminal Cys(X)(2)Cys motifs are not required for copper resistance or transport. Biochem Biophys Res Commun 286:414–418
Strausak D et al (2003) Kinetic analysis of the interaction of the copper chaperone Atox1 with the metal binding sites of the Menkes protein. J Biol Chem 278:20821–20827
Lorinczi E et al (2008) Delivery of the Cu-transporting ATPase ATP7B to the plasma membrane in Xenopus oocytes. Biochim Biophys Acta 1778:896–906
Tsivkovskii R, MacArthur BC, Lutsenko S (2001) The Lys1010–Lys1325 fragment of the Wilson’s disease protein binds nucleotides and interacts with the N-terminal domain of this protein in a copper-dependent manner. J Biol Chem 276:2234–2242
Lutsenko S, Efremov RG, Tsivkovskii R, Walker JM (2002) Human copper-transporting ATPase ATP7B (the Wilson’s disease protein): biochemical properties and regulation. J Bioenerg Biomembr 34:351–362
Veldhuis NA et al (2009) Phosphorylation regulates copper-responsive trafficking of the Menkes copper transporting P-type ATPase. Int J Biochem Cell Biol (Epub ahead of print)
Valverde RH et al (2008) Cyclic AMP-dependent protein kinase controls energy interconversion during the catalytic cycle of the yeast copper-ATPase. FEBS Lett 582:891–895
Acknowledgments
This work was funded by National Institutes of Health grant R01 DK071865 to S.L. A.N.B. is a recipient of NRSA award F32DK077429.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article will be printed in the upcoming Journal of Biological Inorganic Chemistry special issue Cell Biology of Copper.
Rights and permissions
About this article
Cite this article
Barry, A.N., Shinde, U. & Lutsenko, S. Structural organization of human Cu-transporting ATPases: learning from building blocks. J Biol Inorg Chem 15, 47–59 (2010). https://doi.org/10.1007/s00775-009-0595-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-009-0595-4