Abstract
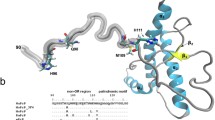
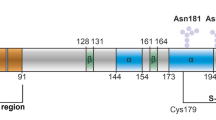
The prion protein (PrPc) is a copper-binding glycoprotein that can misfold into a β-sheet-rich and pathogenic isoform (PrPsc) leading to prion diseases. The first non-mammalian PrPc was identified in chicken and it was found to keep many structural motifs present in mammalian PrPc, despite the low sequence identity (approximately 40%) between the two primary structures. The present paper describes the synthesis and the coordination properties of some hexapeptide fragments (namely, PHNPGY , HNPGYP and NPGYPH) as well as a bishexapeptide (PHNPGYPHNPGY), which encompasses two hexarepeats. The copper(II) complexes were characterized by means of potentiometric, UV–vis, circular dichroism and electron paramagnetic resonance techniques. We also report the synthesis of three hexapeptides (PHNPGF, HNPGFP and NPGFPH), in which one tyrosine was replaced by phenylalanine as well as two bishexapeptides in which either one (PHNPGFPHNPGY and PHNPGYPHNPGF), or two tyrosines were replaced by phenylalanine, in order to check whether tyrosine was involved in copper(II) binding. Overall, the results indicate that the major copper(II) species formed by the chicken PrP dodecapeptides are stabler than the analogous species reported for the peptide fragments containing two octarepeat peptides from the mammalian prion protein. It is concluded that the presence of four prolyl residues, that are break points in copper coordination, induces the metal-assisted formation of macrochelates as well as the formation of binuclear species. Furthermore, it has been shown that the phenolic group is directly involved in the formation of copper binuclear species.









Similar content being viewed by others
References
Prusiner SB (1997) Science 278:245–251
Prusiner SB (1998) Cell 93:337–348
Brown DR, Qin KF, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, Vonbohlen A, Schulzschaeffer W, Giese A, Westaway D, Kretzschmar H (1997) Nature 390:684–687
Lehmann S (2002) Curr Opin Chem Biol 6:187–192
Vassallo N, Herms J (2003) J Neurochem 86:538–544
Brown DR (2003) J Neurochem 87:377–385
Viles JH, Cohen FE, Prusiner SB, Goodin DB, Wright PE, Dyson HJ (1999) Proc Natl Acad Sci USA 96:2042–2046
Whittal RM, Ball HL, Cohen FE, Burlingame AL, Prusiner SB, Baldwin MA (2000) Protein Sci 9:332–343
Millhauser GL (2004) Acc Chem Res 37:79–85
Jackson GS, Murray I, Hosszu LLP, Gibbs N, Waltho JP, Clarke AR, Collinge J (2001) Proc Natl Acad Sci USA 98:8531–8535
Kramer ML, Kratzin HD, Schmdt B, Romer A, Windl O, Liemann S, Hornemann S, Kretzschmar H (2001) J Biol Chem 276:16711–16719
Garnett AP, Viles JH (2003) J Biol Chem 278:6795–6802
Luczkowski M, Kozlowski H, Stawikowski M, Rolka K, Gaggelli E, Valensin D, Valensin G (2002) J Chem Soc Dalton Trans 2269–2275
Valensin D, Luczkowski M, Mancini FM, Legowska A, Gaggelli E, Valensin G, Rolka K, Kozlowski H (2004) J Chem Soc Dalton Trans 1284–1293
Harris DA, Falls DL, Johnson FA, Fischbach GD (1991) Proc Natl Acad Sci USA 88:7664–7668
Gabriel JM, Oesch B, Kretzschamer H, Scott M, Prusiner SB (1992) Proc Natl Acad Sci USA 89:9097–9101
Calzolai L, Lysek DA, Perez DR, Guntert P, Wuthrich K (2005) Proc Natl Acad Sci USA 102:651–655
Marcotte EM, Eisenberg D (1999) Biochemistry 38:667–676
Hornshaw MP, McDermott JR, Candy JM (1995) Biochem Biophys Res Commun 20:621–629
Hornshaw MP, McDermott JR, Candy JM, Lakey JH (1995) Biochem Biophys Res Commun 214:993–999
Pettit LD, Robbins RA (1995) In: Berthon G (ed) Handbook of metal–ligand interactions in biological fluids. Dekker, New York, pp 636–656
Bonomo RP, La Mendola D, Maccarrone G, Pappalardo G, Rizzarelli E (2003) J Inorg Biochem 96:190
Bonomo RP, Impellizzeri G, Pappalardo G, Rizzarelli E, Tabbì G (2000) Chem Eur J 6:4195–4202
Stanczak P, Luczkowski M, Juszczyk P, Grzonka Z, Kozlowski H (2004) J Chem Soc Dalton Trans 2102–2107
Arena G, Calì R, Rizzarelli E, Sammartano S (1976) Thermochim Acta 16:315
Gans P, Sabatini A, Vacca A (1996) Talanta 43:1739–1753
Bonomo RP, Calì R, Cucinotta V, Impellizzeri G, Rizzarelli E (1986) Inorg Chem 25:1641–1646
Gampp H, Maeder M, Meyer CJ, Zuberbuhler D (1985) Talanta 32:257–264
Bonomo RP, Bruno V, Conte E, De Guidi G, La Mendola D, Maccarroine G, Nicoletti F, Rizzarelli E, Sortino S, Vecchio G (2003) Dalton Trans 4406–4415
Hefford RJW, Pettit LD (1981) J Chem Soc Dalton Trans 1331–1335
Pettit LD, Steel I, Kovalik T, Kozlowski H, Bataille M (1985) J Chem Soc Dalton Trans 1201–1205
Livera C, Pettit LD, Bataille M, Krembel J, Bal W, Kozlowski H (1988) J Chem Soc Dalton Trans 1357–1360
Kiss T, Szucs Z (1986) J Chem Soc Dalton Trans 2443–2447
Kiss T (1987) J Chem Soc Dalton Trans 1263–1265
Brahmachari SK, Bhat TN, Sudhakar V, Vijayan M, Rapaka SR, Bhatnagar RS, Ananthanarayanan VS (1981) J Am Chem Soc 103:1703–1708
Zahn R, Liu A, Luhrs T, Riek R, Von Schroetter C, Lopez Garcia F, Billeter M, Calzolai L, Wider G, Wuthrich K (2000) Proc Natl Acad Sci USA 97:145–150
Lopez Garcia F, Zahn R, Riek R, Wuthrich K (2000) Proc Natl Acad Sci USA 97:8334–8339
Koslowski A, Sreerama N, Woody RW (2000) In: Berova N, Nakanishi K, Woody RW (eds) Circular dichroism. Wiley-VCH, New York
Woody RW (1996) In: Fasman GD (ed) Circular dichroism and the conformational analysis of biomolecules. Plenum, New York
Feller SM, Ren R, Hanafusa H, Baltimore D (1994) Trends Biochem Sci 19:453–458
Simon JA, Schreiber SL (1995) Chem Biol 2:53–60
Lee CH, Saksela K, Mirza UA, Chait BT, Kuriyan J (1996) Cell 85:931–942
Woody RW (1992) Adv Biophys Chem 2:37–79
Bienkiewicz E, Woody A-Y, Woody RW (2000) J Mol Biol 297:119–133
Dalcol I, Pons M, Ludevid MD, Giralt E (1996) J Org Chem 61:6775–6782
Petrella EC, Machesky LM, Kaiser DA, Polaard TD (1996) Biochemistry 35:16535–16543
Park SH, Shalongo W, Stellwagen E (1997) Protein Sci 6:1694–1700
Kelly MA, Chellgren BW, Rucker AL, Troutman JM, Fried MG, Fried A-F, Creamer TP (2001) Biochemistry 40:14376–14383
Brookes G, Pettit LD (1975) J Chem Soc Dalton Trans 2106–2112
Pettit LD, Steel I, Formicka-Kozlowki G, Kozlowski H, Tatarowski T, Bataille M (1985) J Chem Soc Dalton Trans 535–539
Toni M, Massimino ML, Griffoni C, Salvato B, Tomasi V, Spisni E (2005) FEBS Lett 579:741–744
Matthews D, Cooke BC (2003) Rev Sci Tech 22:283–296
Lysek DA, Wuthrich K (2004) Biochemistry 43:10393–10399
Kovacs GG, Trabattoni G, Hainfellner JA, Ironside JW, Kinght RS, Budka H (2002) J Neurol 249:1567–1582
Acknowledgements
This work was in part supported by the University of Catania, CNR Rome, and MIUR (PRIN-2003031424 and grant no.196 D.M. 1105/2002).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
La Mendola, D., Bonomo, R.P., Impellizzeri, G. et al. Copper(II) complexes with chicken prion repeats: influence of proline and tyrosine residues on the coordination features. J Biol Inorg Chem 10, 463–475 (2005). https://doi.org/10.1007/s00775-005-0659-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-005-0659-z