Abstract
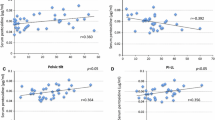
The aim of this study was to investigate the relationship between the radiographic severity of lumbar spondylosis (LS) and serum bone metabolic markers. A total of 681 individuals volunteered for this study (269 men, 412 women; age: 54.9 ± 14.3; body mass index [BMI]: 23.1 ± 3.3 kg/m2). Lateral lumbar radiographs were evaluated in each intervertebral section (L1/2 to L5/S1) using the Kellgren-Lawrence grade (KL). If at least one intervertebral section was graded as KL 2 or greater, the participants were considered to have LS. The summation of each section of intervertebral section was used as the radiographic severity value of LS. In addition, bone status was evaluated with an osteo-sono assessment index (OSI) at the calcaneus. Serum bone alkaline phosphatase (μg/mL), N-telopeptide of type I collagen (nMBCE/L), and pentosidine (pmol/mL) concentrations were examined and used as the bone metabolism index. Stepwise multiple linear regression analysis was conducted with the radiographic severity value of LS as the dependent variable and age, sex, BMI, OSI, and the value of serum bone metabolic markers as the independent variables. The total number of LS participants was 470 (69.0 %); the frequency of LS was higher in men (n = 198) than in women (n = 272; P = 0.036, χ 2 test). The mean severity value of LS was 7.1 ± 4.4, and the mean value of pentosidine was 120.7 ± 54.8 pmol/mL. On multiple regression analysis, age (B = 0.190, β = 0.611), sex (men = 1, women = 2; B = −0.900, β = −0.099), BMI (B = 0.185, β = 0.136), and pentosidine (B = 0.009, β = 0.115) were significantly correlated with the severity of LS. Serum pentosidine concentration was positively correlated with the radiographic severity of LS in this cross-sectional study.


Similar content being viewed by others
References
Kong MH, Morishita Y, He W et al (2009) Lumbar segmental mobility according to the grade of the disc, the facet joint, the muscle, and the ligament pathology by using kinetic magnetic resonance imaging. Spine (Phila Pa 1976) 34:2537–2544
Barrey C, Jund J, Noseda O, Roussouly P (2007) Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J 16:1459–1467
Kalichman L, Li L, Hunter DJ, Been E (2011) Association between computed tomography-evaluated lumbar lordosis and features of spinal degeneration, evaluated in supine position. Spine J 11:308–315
de Schepper EI, Damen J, van Meurs JB et al (2010) The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976) 35:531–536
Kalichman L, Hunter DJ (2007) Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum 37:69–80
Schneck CD (1985) The anatomy of lumbar spondylosis. Clin Orthop Relat Res 193:20–37
Yoshimura N, Muraki S, Oka H et al (2009) Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab 9:620–628
Muraki S, Akune T, Oka H et al (2012) Incidence and risk factors for radiographic lumbar spondylosis and lower back pain in Japanese men and women: the ROAD study. Osteoarthr Cartil 20:712–718
Miyakoshi N, Itoi E, Murai H, Wakabayashi I, Ito H, Minato T (2003) Inverse relation between osteoporosis and spondylosis in postmenopausal women as evaluated by bone mineral density and semiquantitative scoring of spinal degeneration. Spine (Phila Pa 1976) 28:492–495
Salo S, Leinonen V, Rikkonen T, Vainio P, Marttila J, Honkanen R et al (2014) Association between bone mineral density and lumbar disc degeneration. Maturitas 79:449–455
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21:195–214
Numasawa T, Ono A, Wada K et al (2012) Simple foot tapping test as a quantitative objective assessment of cervical myelopathy. Spine (Phila Pa 1976) 37:108–113
Kumagai G, Ono A, Numasawa T et al (2014) Association between roentgenographic findings of the cervical spine and neck symptoms in a Japanese community population. J Orthop Sci 19:390–397
Sasaki E, Ono A, Yokoyama T et al (2014) Prevalence and symptom of ossification of posterior longitudinal ligaments in the Japanese general population. J Orthop Sci 19:405–411
Warner WL, Meeker M, Eells K (1949) Social class in America. Science Research, New York
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Orimo H, Shiraki M, Hayashi Y et al (1994) Effects of lα-Hydroxyvitamin D3 on lumbar bone mineral density and vertebral fractures in patients with postmenopausal osteoporosis. Calcif Tissue Int 54:370–376
Odetti P, Fogarty J, Sell DR, Monnier VM (1992) Chromatographic quantitation of plasma and erythrocyte pentosidine in diabetic and uremic subjects. Diabetes 41:153–159
Peel NF, Barrington NA, Blumsohn A, Colwell A, Hannon R, Eastell R (1995) Bone mineral density and bone turnover in spinal osteoarthrosis. Ann Rheum Dis 54:867–871
Ichchou L, Allali F, Rostom S et al (2010) Relationship between spine osteoarthritis, bone mineral density and bone turn over markers in post menopausal women. BMC Women’s Health 10:25
Papakitsou EF, Margioris AN, Dretakis KE et al (2004) Body mass index (BMI) and parameters of bone formation and resorption in postmenopausal women. Maturitas 47:185–193
Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11:337–349
Chaki O, Yoshikata I, Kikuchi R et al (2000) The predictive value of biochemical markers of bone turnover for bone mineral density in postmenopausal Japanese women. J Bone Miner Res 15:1537–1544
Scariano JK, Garry PJ, Montoya GD, Wilson JM, Baumgartner RN (2001) Critical differences in the serial measurement of three biochemical markers of bone turnover in the sera of pre- and postmenopausal women. Clin Biochem 34:639–644
Sugiyama S, Miyata T, Ueda Y et al (1998) Plasma levels of pentosidine in diabetic patients: an advanced glycation end product. J Am Soc Nephrol 9:1681–1688
Miyata T, Iida Y, Horie K, Cai Z, Sugiyama S, Maeda K (1996) Pathophysiology of advanced glycation end-products in renal failure. Nephrol Dial Transplant 11:27–30
Koschinsky T, He CJ, Mitsuhashi T et al (1997) Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA 94:6474–6479
Yamagishi S (2011) Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol 46:217–224
Loeser RF, Yammani RR, Carlson CS et al (2005) Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheum 52:2376–2385
Steenvoorden MM, Huizinga TW, Verzijl N et al (2006) Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum 54:253–263
Rasheed Z, Akhtar N, Haqqi TM (2011) Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology (Oxford) 50:838–851
Chen YJ, Sheu ML, Tsai KS, Yang RS, Liu SH (2013) Advanced glycation end products induce peroxisome proliferator-activated receptor γ down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products. PLoS One 8:e66611
Vos PA, Welsing PM, deGroot J et al (2013) Skin pentosidine in very early hip/knee osteoarthritis (CHECK) is not a strong independent predictor of radiographic progression over 5 years follow-up. Osteoarthr Cartil 21:823–830
Abbas J, Hamoud K, Masharawi YM et al (2010) Ligamentum flavum thickness in normal and stenotic lumbar spines. Spine (Phila Pa 1976) 35:1225–1230
Acknowledgments
We are exceedingly grateful to all participants in the Iwaki Health Promotion Project and to the staff of our department who conducted interviews and collected data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests to declare.
Authors’ roles
Study design: DC. Study conduct: DC and ES. Data collection: DC and GK, and IT. Data analysis: DC, GK, and ES. Data interpretation: DC, KW, and ES. Drafting manuscript: DC. Revising manuscript: KW, TT, GK, and ES. Approving final version of manuscript: SN and YI.
Funding
The Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 18200044), the Japanese Society for the Promotion of Science (No. 21500676), Karoji Memorial Fund for Medical Research (No. 5310139302), and JOA-Subsidized Science Project Research from the Japanese Orthopaedic Association.
About this article
Cite this article
Chiba, D., Wada, K., Tanaka, T. et al. Serum pentosidine concentration is associated with radiographic severity of lumbar spondylosis in a general Japanese population. J Bone Miner Metab 35, 65–72 (2017). https://doi.org/10.1007/s00774-015-0727-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0727-6