Abstract
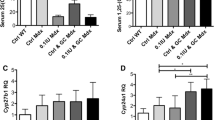
Glucocorticoids cause secondary osteoporosis and myopathy, characterized by type II muscle fiber atrophy. We examined whether a new vitamin D3 analogue, eldecalcitol, could inhibit glucocorticoid-induced osteopenia or myopathy in rats, and also determined the effects of prednisolone (PSL) and/or eldecalcitol on muscle-related gene expression. Six-month-old female Wistar rats were randomized into four groups: PSL group (10 mg/kg PSL); E group (0.05 µg/kg eldecalcitol); PSL + E group; and control group. PSL, eldecalcitol, and vehicles were administered daily for 2 or 4 weeks. Right calf muscle strength, muscle fatigue, cross-sectional areas (CSAs) of left tibialis anterior muscle fibers, and bone mineral density (BMD) were measured following administration. Pax7, MyoD, and myogenin mRNA levels in gastrocnemius muscles were also determined. Muscle strength was significantly higher in the PSL + E group than in the PSL group (p < 0.05) after 4 weeks, but not after 2 weeks. No significant difference in muscle fatigue was seen between groups at 2 or 4 weeks. CSAs of type II muscle fibers were significantly larger in the E group and the PSL + E group than in the PSL group at 4 weeks (p = 0.0093, p = 0.0443, respectively). Eldecalcitol treatment for 4 weeks maintained the same BMD as the PSL + E group. After 2 weeks, but not 4 weeks, eldecalcitol treatment significantly increased Pax7 and myogenin mRNA expression in gastrocnemius muscle, and PSL also stimulated myogenin expression. Eldecalcitol appears to increase muscle volume and to protect against femur BMD loss in PSL-administered rats, and it may also stimulate myoblast differentiation into early myotubes.


Similar content being viewed by others
References
da Costa JA, Ribeiro A, Bogas M, Costa L, Varino C, Lucas R, Rodrigues A, Araújo D (2009) Mortality and functional impairment after hip fracture: a prospective study in a Portuguese population. Acta Reumatol Port 34:618–626
Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ 3rd (1993) Population-based study of survival after osteoporotic fractures. Am J Epidemiol 137:1001–1005
Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G (2012) Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 31:652–658
Chan BK, Marshall LM, Winters KM, Faulkner KA, Schwartz AV, Orwoll ES (2007) Incident fall risk and physical activity and physical performance among older men: the Osteoporotic Fractures in Men Study. Am J Epidemiol 165:696–703
Pan HH, Li CY, Chen TJ, Su TP, Wang KY (2014) Association of polypharmacy with fall-related fractures in older Taiwanese people: age- and gender-specific analyses. BMJ Open 4:e004428
Keller K, Engelhardt M (2014) Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J 3:346–350
Johnell K, Fastbom J (2008) Multi-dose drug dispensing and inappropriate drug use: a nationwide register-based study of over 700,000 elderly. Scand J Prim Health Care 26:86–91
Pereira RM, Freire de Carvalho J (2011) Glucocorticoid-induced myopathy. Joint Bone Spine 78:41–44
Larsson L, Sjödin B, Karlsson J (1978) Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand 103:31–39
Schakman O, Gilson H, Thissen JP (2008) Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 197:1–10
Skelton DA, Kennedy J, Rutherford OM (2002) Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing 31:119–125
Gallagher JC, Fowler SE, Detter JR, Sherman SS (2001) Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab 86:3618–3628
Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, Wong JB (2004) Effect of Vitamin D on falls: a meta-analysis. JAMA 291:1999–2006
Dukas L, Schacht E, Mazor Z, Stähelin HB (2005) Treatment with alfacalcidol in elderly people significantly decreases the high risk of falls associated with a low creatinine clearance of <65 ml/min. Osteoporos Int 16:198–203
Kasukawa Y, Miyakoshi N, Maekawa S, Nozaka K, Noguchi H, Shimada Y (2010) Effects of alfacalcidol on muscle strength, muscle fatigue, and bone mineral density in normal and ovariectomized rats. Biomed Res 31:273–279
Miyakoshi N, Sasaki H, Kasukawa Y, Kamo K, Shimada Y (2010) Effects of a vitamin D analog, alfacalcidol, on bone and skeletal muscle in glucocorticoid-treated rats. Bio Med Res 31:329–336
Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M, Nakamura T (2011) A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures—a randomized, active comparator, double-blind study. Bone (NY) 49:605–612
O’Donnell S, Moher D, Thomas K, Hanley DA, Cranney A (2008) Systematic review of the benefits and harms of calcitriol and alfacalcidol for fractures and falls. J Bone Miner Metab 26:531–542
Nakamura T, Takano T, Fukunaga M, Shiraki M, Matsumoto T (2013) Eldecalcitol is more effective for the prevention of osteoporotic fractures than alfacalcidol. J Bone Miner Metab 31:417–422
Hagino H, Takano T, Fukunaga M, Shiraki M, Nakamura T, Matsumoto T (2013) Eldecalcitol reduces the risk of severe vertebral fractures and improves the health-related quality of life in patients with osteoporosis. J Bone Miner Metab 31:183–189
Ito M, Nakamura T, Fukunaga M, Shiraki M, Matsumoto T (2011) Effect of eldecalcitol, an active vitamin D analog, on hip structure and biomechanical properties: 3D assessment by clinical CT. Bone (NY) 49:328–334
Harada S, Takeda S, Uno A, Takahashi F, Saito H (2010) Eldecalcitol is less effective in suppressing parathyroid hormone compared to calcitriol in vivo. J Steroid Biochem Mol Biol 121:281–283
Sasaki H, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Kamo K, Shimada Y (2009) Muscle strength and fatigue in glucocorticoid-treated rats. Akita J Med 36:195–202
Clarke HH, Shay CT, Mathews DK (1954) Strength decrement of elbow flexor muscles following exhaustive exercise. Arch Phys Med Rehabil 35:560–567
Murata K, Yano E (2002) Medical statistics for evidence-based medicine with SPBS user’s guide. Nankodo, Tokyo
Compston J (2010) Management of glucocorticoid-induced osteoporosis. J Nat Rev Rheumatol 6:82–88
Saito H, Kishimoto K, Okuno H, Tanaka M, Itoi E (2013) Effect of eldecalsitol on C2C12 cell. (in Japanese). Clin Calcium 23:142
Tanaka K, Kanazawa I, Yamaguchi T, Yano S, Kaji H, Sugimoto T (2014) Active vitamin D possesses beneficial effects on the interaction between muscle and bone. Biochem Biophys Res Commun. doi:10.1016/j.bbrc.2014.05.145
Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786
Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I, Nabeshima Y (1993) Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature (Lond) 364:532–535
Smith CK 2nd, Janney MJ, Allen RE (1994) Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol 159:379–385
Perez-Ruiz A, Ono Y, Gnocchi VF, Zammit PS (2008) β-Catenin promotes self-renewal of skeletal-muscle satellite cells. J Cell Sci 121:1373–1382
Ceglia L, Rivas DA, Pojednic RM, Price LL, Harris SS, Smith D, Fielding RA, Dawson-Hughes B (2013) Effects of alkali supplementation and vitamin D insufficiency on rat skeletal muscle. Endocrine 44:454–464
Lieu FK, Powers SK, Herb RA, Criswell D, Martin D, Wood C, Stainsby W, Chen CL (1993) Exercise and glucocorticoid-induced diaphragmatic myopathy. J Appl Physiol 75:763–771
Dong Y, Pan JS, Zhang L (2013) Myostatin suppression of Akirin1 mediates glucocorticoid-induced satellite cell dysfunction. PLoS One 8:e58554
te Pas MF, de Jong PR, Verburg FJ (2000) Glucocorticoid inhibition of C2C12 proliferation rate and differentiation capacity in relation to mRNA levels of the MRF gene family. Mol Biol Rep 27:87–98
de Rekeneire N, Visser M, Peila R, Nevitt MC, Cauley JA, Tylavsky FA, Simonsick EM, Harris TB (2003) Is a fall just a fall: correlates of falling in healthy older persons. The Health, Aging and Body Composition Study. J Am Geriatr Soc 51:841–846
Iwamoto J, Sato Y (2014) Eldecalcitol improves chair-rising time in postmenopausal osteoporotic women treated with bisphosphonates. Ther Clin Risk Manag 10:51–59
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant No. 25462284).
Conflict of interest
Drs. Kinoshita, Miyakoshi, Kasukawa, Segawa, Ohuchi, Fujii, Sato, and Shimada have no conflicts of interest. Drs. Shiraishi and Sakai are current employees of Chugai Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kinoshita, H., Miyakoshi, N., Kasukawa, Y. et al. Effects of eldecalcitol on bone and skeletal muscles in glucocorticoid-treated rats. J Bone Miner Metab 34, 171–178 (2016). https://doi.org/10.1007/s00774-015-0664-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0664-4