Abstract
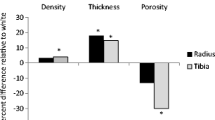
Few childhood studies have investigated racial differences in volumetric bone mineral density (vBMD), bone geometry, and bone strength indices measured by three-dimensional bone imaging. The purpose of this study was to compare trabecular and cortical bone parameters at the radius and tibia between late adolescent white and black females using peripheral quantitative computed tomography (QCT). White (n = 25) and black females (n = 25), 18–19 years of age, were pair-matched for age, height, and fat-free soft tissue mass. Peripheral QCT scans were obtained at the 4% (trabecular bone), 20% (cortical bone), and 66% [muscle cross-sectional area (CSA)] sites from the distal metaphyses. Bone strength was determined from vBMD and bone geometry to calculate bone strength index (BSI; trabecular site) and polar strength–strain index (SSI; cortical site). Radial SSI was not different between groups; however, blacks had greater radial BSI (P = 0.02) than whites. After adjustment for the longer forearm in blacks, the greater radial BSI in blacks no longer remained. At the tibia, blacks versus whites had greater bone strength at the trabecular and cortical bone sites (BSI, P = 0.03; SSI, P = 0.04, respectively). When controlling for differences in tibial length and muscle CSA, the higher estimates of bone strength persisted in blacks versus whites (BSI, P = 0.01; SSI, P = 0.02). Our data suggest that when differences in body size are considered, late adolescent black versus white females have a stronger bone profile, due to greater bone geometry and vBMD, at the trabecular and cortical regions of the tibia but not at the radius.
Similar content being viewed by others
References
Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM (2005) Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res 20:185–194
Li JY, Specker BL, Ho ML, Tsang RC (1989) Bone mineral content in black and white children 1 to 6 years of age. Early appearance of race and sex differences. Am J Dis Child 143:1346–1349
Rupich RC, Specker BL, Lieuw AFM, Ho M (1996) Gender and race differences in bone mass during infancy. Calcif Tissue Int 58:395–397
Bell NH, Shary J, Stevens J, Garza M, Gordon L, Edwards J (1991) Demonstration that bone mass is greater in black than in white children. J Bone Miner Res 6:719–723
Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R (1999) Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab 84:4702–4712
Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA (2007) The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 92:2087–2099
Ettinger B, Sidney S, Cummings SR, Libanati C, Bikle DD, Tekawa IS, Tolan K, Steiger P (1997) Racial differences in bone density between young adult black and white subjects persist after adjustment for anthropometric, lifestyle, and biochemical differences. J Clin Endocrinol Metab 82:429–434
Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG (1991) Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med 325:1597–1600
Wang MC, Aguirre M, Bhudhikanok GS, Kendall CG, Kirsch S, Marcus R, Bachrach LK (1997) Bone mass and hip axis length in healthy Asian, black, Hispanic, and white American youths. J Bone Miner Res 12:1922–1935
Hui SL, Dimeglio LA, Longcope C, Peacock M, McClintock R, Perkins AJ, Johnston CC Jr (2003) Difference in bone mass between black and white American children: attributable to body build, sex hormone levels, or bone turnover? J Clin Endocrinol Metab 88:642–649
Seeman E (1998) Growth in bone mass and size: are racial and gender differences in bone mineral density more apparent than real? J Clin Endocrinol Metab 83:1414–1419
Looker AC (2002) The skeleton, race, and ethnicity. J Clin Endocrinol Metab 87:3047–3050
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone (NY) 14:595–608
Currey J (1999) The design of mineralised tissues for their mechanical functions. J Exp Biol 202:3285–3291
Schonau E (1998) Problems of bone analysis in childhood and adolescence. Pediatr Nephrol 12:420–429
Gilsanz V, Skaggs DL, Kovanlikaya A, Sayre J, Loro ML, Kaufman F, Korenman SG (1998) Differential effect of race on the axial and appendicular skeletons of children. J Clin Endocrinol Metab 83:1420–1427
Wetzsteon RJ, Hughes JM, Kaufman BC, Vazquez G, Stoffregen TA, Stovitz SD, Petit MA (2009) Ethnic differences in bone geometry and strength are apparent in childhood. Bone (NY) 44:970–975
Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS (2010) Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab 95:1681–1689
Frost H (1987) Bone “mass” and the “mechanostat”: a proposal. Anat Rec 219:1–9
Rauch F, Schoenau E (2001) The developing bone: slave or master of its cells and molecules? Pediatr Res 50:309–314
Klein GL, Fitzpatrick LA, Langman CB, Beck TJ, Carpenter TO, Gilsanz V, Holm IA, Leonard MB, Specker BL (2005) The state of pediatric bone: summary of the ASBMR pediatric bone initiative. J Bone Miner Res 20:2075–2081
Petit MA, Beck TJ, Kontulainen SA (2005) Examining the developing bone: what do we measure and how do we do it? J Musculoskelet Neuronal Interact 5:213–224
Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD (2007) Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr 86:1530–1538
Sievanen H, Koskue V, Rauhio A, Kannus P, Heinonen A, Vuori I (1998) Peripheral quantitative computed tomography in human long bones: evaluation of in vitro and in vivo precision. J Bone Miner Res 13:871–882
Muller ME, Webber CE, Bouxsein ML (2003) Predicting the failure load of the distal radius. Osteoporos Int 14:345–352
Martin RB (1991) Determinants of the mechanical properties of bones. J Biomech 24(suppl 1):79–88
Kontulainen SA, Johnston JD, Liu D, Leung C, Oxland TR, McKay HA (2008) Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact 8:401–409
Schiessl HFJ, Ferreti JL, Tysarczqk-Niemeyer G, Willnecker J (1996) Non-invasive bone strength index as analyzed by peripheral quantitative computed tomography (pQCT). In: Schoenau E (ed) Pediatric osteology: new developments in diagnosis and therapy. Elsevier, Amsterdam, pp 141–166
Rittweger J, Beller G, Ehrig J, Jung C, Koch U, Ramolla J, Schmidt F, Newitt D, Majumdar S, Schiessl H, Felsenberg D (2000) Bone–muscle strength indices for the human lower leg. Bone (NY) 27:319–326
Schoenau E, Neu CM, Mokov E, Wassmer G, Manz F (2000) Influence of puberty on muscle area and cortical bone area of the forearm in boys and girls. J Clin Endocrinol Metab 85:1095–1098
Blair SN, Haskell WL, Ho P, Paffenbarger RS Jr, Vranizan KM, Farquhar JW, Wood PD (1985) Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 122:794–804
Washburn RA, Jacobsen DJ, Sonko BJ, Hill JO, Donnelly JE (2003) The validity of the Stanford seven-day physical activity recall in young adults. Med Sci Sports Exerc 35:1374–1380
Cohen J (1988) Statistical power analysis for the behavioral sciences. Academic Press, New York
Schoenau E (2005) From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J Musculoskelet Neuronal Interact 5:232–238
Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, Tosteson T (1994) Racial differences in fracture risk. Epidemiology 5:42–47
Baron JA, Karagas M, Barrett J, Kniffin W, Malenka D, Mayor M, Keller RB (1996) Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology 7:612–618
Harris SS, Wood MJ, Dawson-Hughes B (1995) Bone mineral density of the total body and forearm in premenopausal black and white women. Bone (NY) 16:311S–315S
Saeed I, Carpenter RD, Leblanc AD, Li J, Keyak JH, Sibonga JD, Lang TF (2009) Quantitative computed tomography reveals the effects of race and sex on bone size and trabecular and cortical bone density. J Clin Densitom 12:330–336
Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH (2001) Mechanical loading of diaphyseal bone in vivo: the strain threshold for an osteogenic response varies with location. J Bone Miner Res 16:2291–2297
Iwamoto J, Yeh JK, Aloia JF (1999) Differential effect of treadmill exercise on three cancellous bone sites in the young growing rat. Bone (NY) 24:163–169
Turner CH (2002) Determinants of skeletal fragility and bone quality. J Musculoskelet Neuronal Interact 2:527–528
Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB (1992) Bone gain in young adult women. JAMA 268:2403–2408
Richards RJ, Svec F, Bao W, Srinivasan SR, Berenson GS (1992) Steroid hormones during puberty: racial (black–white) differences in androstenedione and estradiol: the Bogalusa Heart Study. J Clin Endocrinol Metab 75:624–631
Nilsson A, Ohlsson C, Isaksson OG, Lindahl A, Isgaard J (1994) Hormonal regulation of longitudinal bone growth. Eur J Clin Nutr 48(suppl 1):S150–S158 (discussion S158–S160)
Berman DM, Rodrigues LM, Nicklas BJ, Ryan AS, Dennis KE, Goldberg AP (2001) Racial disparities in metabolism, central obesity, and sex hormone-binding globulin in postmenopausal women. J Clin Endocrinol Metab 86:97–103
Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS (2002) National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics 110:911–919
Schiessl H, Frost HM, Jee WS (1998) Estrogen and bone-muscle strength and mass relationships. Bone (NY) 22:1–6
Rauch F, Schoenau E (2008) Peripheral quantitative computed tomography of the proximal radius in young subjects: new reference data and interpretation of results. J Musculoskelet Neuronal Interact 8:217–226
Duan Y, Beck TJ, Wang XF, Seeman E (2003) Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res 18:1766–1774
Seeman E, Delmas PD (2006) Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261
Zemel B, Bass S, Binkley T, Ducher G, Macdonald H, McKay H, Moyer-Mileur L, Shepherd J, Specker B, Ward K, Hans D (2008) Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:59–74
Fricke O, Schoenau E (2005) Examining the developing skeletal muscle: why, what and how? J Musculoskelet Neuronal Interact 5:225–231
Acknowledgments
The authors thank the study subjects for their participation. We also thank Jessica Principe, Veronique Bredas, Ashley Ferira, and Maria Breen for coordinating the project. N.K.P., R.D.L., E.M.L., C.A.B., M.W.H., and D.B.H. were responsible for the study concept and design; N.K.P., E.M.L., R.G.F., and R.D.L. were responsible for the acquisition of the data. N.K.P. and D.B.H. conducted the statistical analyses. N.K.P., R.D.L., E.M.L., R.G.F., and D.B.H. were responsible for the interpretation of the data and drafting the manuscript. All authors contributed to the revision of the manuscript. Funding was received from The University of Georgia Research Foundation and College of Family and Consumer Sciences.
Conflict of interest statement
None of the authors had any personal or financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pollock, N.K., Laing, E.M., Taylor, R.G. et al. Comparisons of trabecular and cortical bone in late adolescent black and white females. J Bone Miner Metab 29, 44–53 (2011). https://doi.org/10.1007/s00774-010-0186-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-010-0186-z