Abstract
The protocol consists of running a native gel with in-gel digestion by proteases, subsequent mass spectrometrical determination of protein sequence and modifications, followed by electro-elution and conformational analysis using melting point and circular dichroism. Finally, the eluted protein is tested for preserved function. Herein, C1 esterase inhibitor is applied on a native gel; in-gel digestion by proteases is carried out and peptides are identified by nano-LC-ESI-CID/ETD-MS/MS using an ion trap for generation of peptide sequences and protein modifications. Protein from replicate bands from the same gel is electro-eluted and used for determination of the melting point and used for circular dichroism analysis. Additional bands from the native gel are either in-gel digested with asparaginase to generate deamidation or PNGase F for deglycosylation, followed by mass spectrometry, conformational and functional studies. Preserved conformation and function of the C1 esterase inhibitor was shown. This protocol can be completed in 1 week.



Similar content being viewed by others
References
Albertini AA et al (2007) Isolation and crystallization of a unique size category of recombinant Rabies virus nucleoprotein-RNA rings. J Struct Biol 158:129–133
Banerji A, Sheffer AL (2009) The spectrum of chronic angioedema. Allergy Asthma Proc 30:11–16
Bock SC et al (1986) Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry 25:4292–4301
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carugati A, Pappalardo E, Zingale LC, Cicardi M (2001) C1-inhibitor deficiency and angioedema. Mol Immunol 38:161–173
Chen WQ et al (2010) Purification of recombinant growth hormone by clear native gels for conformational analyses: preservation of conformation and receptor binding. Amino Acids 39:859–869
Cugno M, Zanichelli A, Foieni F, Caccia S, Cicardi M (2009) C1-inhibitor deficiency and angioedema: molecular mechanisms and clinical progress. Trends Mol Med 15:69–78
Dunahay TG, Staehelin LA (1985) Isolation of photosystem I complexes from octyl glucoside/sodium dodecyl sulfate solubilized spinach thylakoids: characterization and reconstitution into liposomes. Plant Physiol 78:606–613
Evans JR, Anderson JM (1987) Absolute absorption and relative fluorescence excitation spectra of the five major chlorophyll-protein complexes from spinach thylakoid membranes. Biochim Biophys Acta 892:75–82
Ford RC (1987) Investigation of highly stable Photosystem I chlorophyll-protein complexes from the thermophilic cyanobacterium Phormidium laminosum. Biochim Biophys Acta 893:115–125
Ford RC, Picot D, Garavito RM (1987) Crystallization of the photosystem I reaction centre. EMBO J 6:1581–1586
Greenfield NJ (2006) Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 1:2876–2890
Heinz E, Siefermann-Harms D (1981) Are galactolipids integral components of the chlorophyll—protein complexes in spinach thylakoids? FEBS Lett 124:105–111
Kang SU et al (2009) Gel-based mass spectrometric analysis of a strongly hydrophobic GABAA-receptor subunit containing four transmembrane domains. Nat Protoc 4:1093–1102
Kelly SM, Jess TJ, Price NC (2005) How to study proteins by circular dichroism. Biochim Biophys Acta 1751:119–139
Knoetzel J, Braumann T, Grimme LH (1988) Pigment-protein complexes of green algae: improved methodological steps for the quantification of pigments in pigment-protein complexes derived from the green Algae Chlorella and Chlamydomonas. J Photochem Photobiol B Biol 1:475–491
Krause F (2006) Detection and analysis of protein–protein interactions in organellar and prokaryotic proteomes by native gel electrophoresis: (Membrane) protein complexes and supercomplexes. Electrophoresis 27:2759–2781
Lancaster CR, Kroger A, Auer M, Michel H (1999) Structure of fumarate reductase from Wolinella succinogenes at 2.2 A resolution. Nature 402:377–385
Madhavarao CN, Chinopoulos C, Chandrasekaran K, Namboodiri MA (2003) Characterization of the N-acetylaspartate biosynthetic enzyme from rat brain. J Neurochem 86:824–835
Perkins SJ (1993) Three-dimensional structure and molecular modeling of C1 inhibitor. Behring Inst Mitt 93:63–80
Perkins SJ et al (1990) Two-domain structure of the native and reactive centre cleaved forms of C1 inhibitor of human complement by neutron scattering. J Mol Biol 214:751–763
Poetsch A, Neff D, Seelert H, Schagger H, Dencher NA (2000) Dye removal, catalytic activity and 2D crystallization of chloroplast H(+)-ATP synthase purified by blue native electrophoresis. Biochim Biophys Acta 1466:339–349
Schafer E et al (2006) Architecture of active mammalian respiratory chain supercomplexes. J Biol Chem 281:15370–15375
Schafer E, Dencher NA, Vonck J, Parcej DN (2007) Three-dimensional structure of the respiratory chain supercomplex I1III2IV1 from bovine heart mitochondria. Biochemistry 46:12579–12585
Schagger H (2002) Respiratory chain supercomplexes of mitochondria and bacteria. Biochim Biophys Acta 1555:154–159
Schagger H et al (2004) Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J Biol Chem 279:36349–36353
Seelert H, Krause F (2008) Preparative isolation of protein complexes and other bioparticles by elution from polyacrylamide gels. Electrophoresis 29:2617–2636
Seelert H, Dencher NA, Muller DJ (2003) Fourteen protomers compose the oligomer III of the proton-rotor in spinach chloroplast ATP synthase. J Mol Biol 333:337–344
Siefermann-Harms D, Ross JW, Kaneshiro KH, Yamamoto HY (1982) Reconstitution by monogalactosyldiacylglycerol of energy transfer from light-harvesting chlorophyll a/b-protein complex to the photosystems in Triton X-100-solubilized thylakoids. FEBS Lett 149:191–196
Singh J, Wasserman AR (1970) Detection of aggregation and non-destructive disaggregation of membranous proteins using polyacrylamide gel electrophoresis with non-ionic detergents. Biochim Biophys Acta 221:379–382
Tsiotis G, Nitschke W, Haase W, Michel H (1993) Purification and crystallization of Photosystem I complex from a phycobilisome-less mutant of the cyanobacterium Synechococcus PCC 7002. Photosynth Res 35:285–297
Acknowledgments
We acknowledge the contribution by the Verein zur Durchführung der wissenschaftlichen Forschung auf dem Gebiet der Neonatologie und Kinderintensivmedizin “Unser Kind”.
Conflict of interest
The authors declare that they have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
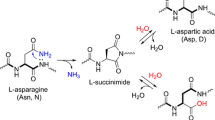
Supplemental Figure 1 10 % SDS-PAGE (a) and 8 % native PAGE (b) separation of C1-1, C1-2, C1-3 and C1-4. C1-1 is C1INH in 10 mM phosphate buffer. C1-2 is C1INH after electro-elution. C1-3 is C1INH after PNGase F treatment. C1-4 is C1INH after asparaginase treatment.In order to show the effect of deamidation and partial deglycosylation, a 10 % SDS-PAGE was cast, all C1INH preparations were run at 50 V for 30 min, 100 V for 1 h 30 min and gels were stained using a Colloidal Coomassie blue staining kit. The band for C1-3 representing partial deglycosylation revealed a remarkable shift towards a lower apparent molecular weight.The band at the position of the boxes (solid line) in the native gel (b) was used for mass spectrometrical analyses. Electro-elution of proteins from an unstained gel run in parallel was carried out from the position as indicated by boxes (dashed). Several bands on the native gel may reflect splice variants, modifications including glycosylation or aggregates.
Supplementary Figure 2(a) The D-Tube has two membrane windows with specific molecular weight cut-off. Put gel band into D-Tube and add buffer. Electrophorese to elute the sample from gel. Put the D-tube into dialysis tank and change to appropriate buffer. (b) Put filter device into provided tube. Add sample into the filter device and cap. Centrifuge at 14,000×g for 10-30 min depending on volume. Immediately separate filter device and reversely put into another tube. 1,000×g for 2 min to spin down the concentrated solution.
Rights and permissions
About this article
Cite this article
Chen, WQ., Karnaukhova, E. & Lubec, G. The use of native gels for the concomitant determination of protein sequences and modifications by mass spectrometry with subsequent conformational and functional analysis of native proteins following electro-elution. Amino Acids 44, 1381–1389 (2013). https://doi.org/10.1007/s00726-013-1477-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1477-1