Abstract
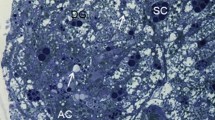
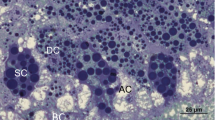
The cave cricket Troglophilus neglectus regularly overwinters for 4–5 months in hypogean habitats. Winter dormancy is a natural starvation period, providing the opportunity to study autophagy under natural conditions. We aimed to evaluate the autophagic activity in adipocytes and urocytes of the fat body in three time frames: directly before overwintering, in the middle of dormancy, and at its end. For this purpose, we sampled individuals in caves. The cell ultrastructure was studied by transmission electron microscopy (TEM) and the abundance of autophagosomes by immunofluorescence microscopy (IFM), applying the widely used, specific immunolabeling marker microtubule-associated protein 1 light chain 3 (LC3). Before overwintering, TEM revealed scarce autophagosomes and residual bodies in the adipocytes and none in the urocytes. Congruently, IFM showed a very limited or no reaction. In the middle and at the end of overwintering, in both cell types, phagophores, autophagosomes, autolysosomes, and residual bodies were identified by TEM, while LC3 immunolabeling for detecting autophagosomes showed a conspicuous positive reaction. Both methods revealed that there were no significant differences between the sexes in any time frame. Minimal autophagic activity was detected before the winter dormancy, and it gradually intensified till the end of overwintering, probably because reserve proteins in protein granula are not composed of all the required amino acids. We conclude that in T. neglectus, autophagy is a substantial response to starvation and supports homeostatic processes during winter dormancy by supplying cells with nutrients.






Similar content being viewed by others
References
Arrese EL, Soulages JL (2010) Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55:207–225
Baehrecke EH (2003) Autophagic programmed cell death in Drosophila (review). Cell Death Differ 10(9):940–945
Barth JM, Szabad J, Hafen E, Kohler K (2011) Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ 18:915–924
Beaulaton J, Lockshin RA (1977) Ultrastructural study of the normal degeneration of the intersegmental muscles of Antheraea polyphemus and Manduca sexta (Insecta, Lepidoptera) with particular reference to cellular autophagy. J Morphol 154(1):39–57
Berg TO, Fengsrud M, Strǿmhaug PE, Berg T, Seglen PO (1998) Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem 273:21883–21892
Brandão AS, Amaral JB, Rezende-Teixeira P, Hartfelder K, Siviero F, Machado-Santelli GM (2014) Cell death and tissue reorganization in Rhynchosciara americana (Sciaridae: Diptera) metamorphosis and their relation to molting hormone titers. Arthropod Struct Dev 43(5):511–522
Cohen E (2003) Fat body. In: Resh VH, Cardé RT (eds) Encyclopedia of insects. Academic, Amsterdam, pp 407–409
Cooper GM, Hausman RE (2009) Protein synthesis, processing, and regulation. In: Cooper GM, Hausman RE (eds) The cell. A molecular approach. ASM Press, Washington, pp 309–352
Eskelinen E-L (2008) Fine structure of the autophagosome. In: Deretic V (ed) Methods in molecular biology, Autophagosome and phagosome. Humana Press, pp 11–28
Franzetti E, Huang ZJ, Shi YX, Xie K, Deng XJ, Li JP, Li QR, Yang WY, Zeng WN, Casartelli M, Deng HM, Cappellozza S, Grimaldi A, Xia Q, Feng Q, Cao Y, Tettamanti G (2012) Autophagy precedes apoptosis during the remodeling of silkworm larval midgut. Apoptosis 17(3):305–324
Gaino E, Mazzini M (1995) Ultrastructural organization of the fat body in three species of mayfly (Ephemeroptera). In: Korkum L, Ciborowski J (eds) Current directions in research on Ephemeroptera. Canadian Scholar, Toronto, pp 347–357
Gioacchino M, Petrarca C, Perrone A, Farina M, Sabbioni E, Hartung T, Martino S, Esposito DL, Lotti LV, Mariani-Constantini R (2008) Autophagy as an ultrastructural marker of heavy metal toxicity in human cord blood hematopoietic stem cells. Sci Total Environ 392:50–58
Hahn DA, Denlinger DL (2011) Energetics of insect diapause. Annu Rev Entomol 56:103–121
Hyatt AD, Marshall AT (1985) Ultrastructure and cytochemistry of the fat body of Periplaneta americana (Dictyoptera: Blattidae). Int J Insect Morphol Embryol 14:131–141
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19(21):5720–5728
Khoa DB, Takeda M (2012) Expression of autophagy 8 (Atg8) and its role in the midgut and other organs of the greater wax moth, Galleria mellonella, during metamorphic remodelling and under starvation. Insect Mol Biol 21(5):473–487
King-Jones K, Thummel CS (2005) Developmental biology. Less steroids make bigger flies. Science 310:630–631
Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y (1999) Formation process of autophagosome is traced with Apg8/Aut7p in Yeast. J Cell Biol 147:435–446
Klionsky DJ, Abdalla FC, Zuckerbraun B (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8(4):445–544
Kondomerkos DJ, Kalamidas SA, Kotoulas OB (2004) An electron microscopic and biochemical study of the effects of glucagon on glycogen autophagy in the liver and heart of newborn rats. Microsc Res Tech 63:87–93
Kroemer G, Galluzzi L, Vandenabeele P et al (2009) Classification of cell death: recommendations of the nomenclature committee on cell death. Cell Death Differ 16(1):3–11
Lionaki E, Markaki M, Tavernarakis N (2013) Autophagy and ageing: insights from invertebrate model organisms. Ageing Res Rev 12:413–428
Lipovšek S, Novak T, Janžekovič F, Pabst MA (2011) Role of the fat body in the cave crickets Troglophilus cavicola and Troglophilus neglectus (Rhaphidophoridae, Saltatoria) during overwintering. Arthropod Struct Dev 40(1):54–63
Lipovšek S, Janžekovič F, Novak T (2014) Autophagic activity in the midgut gland of the overwintering harvestmen Gyas annulatus (Phalangiidae, Opiliones). Arthropod Struct Dev 43:493–500
Lipovšek S, Novak T, Janžekovič F, Leitinger G (2015) Changes in the midgut diverticula in the harvestmen Amilenus aurantiacus (Phalangiidae, Opiliones) during winter diapause. Arthropod Struct Dev. doi:10.1016/j.asd.2014.12.002
Locke M (1998) The fat body. In: Harrison FW, Locke M (eds) Microscopic anatomy of invertebrates, vol 11B, Insecta. Wiley-Liss, New York, pp 641–686
Locke M, Collins JV (1968) Protein uptake into multivesicular bodies and storage granules in the fat body of an insect. J Cell Biol 36(3):453–483
Malagoli D, Abdalla FC, Cao Y, Feng Q, Fujisaki K, Gregorc A, Matsuo T, Nezis IP, Papassideri IS, Sass M, Silva-Zacarin ECM, Tettamanti G, Umemiya-Shirafuji R (2010) Autophagy and its physiological relevance in arthropods: current knowledge and perspectives (review). Autophagy 6(5):575–588
Martinet W, De Bie M, Schrijvers DM, De Meyer GRY, Herman AG, Kockx MM (2004) 7-ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24:2296–2301
Maurizii MG, Mazzini M, Giorgi F (1992) Structural modifications of the fat body in the stick insects Bacillus rossius during larval development. B Zool 59:387–394
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873
Mizushima N, Ohsumi Y, Yoshimori T (2002) Autophagosome formation in mammalian cells. Cell Struct Funct 27:421–429
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075
Munafo DB, Colombo MI (2001) A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J Cell Sci 114:3619–3629
Nezis IP (2012) Selective autophagy in Drosophila. Review article. Int J Cell Biol, Article ID 146767:9. doi:10.1155/2012/146767
Novak T, Janžekovič F, Lipovšek S (2013) Contribution of non-troglobiotic terrestrial invertebrates to carbon input in hypogean habitats. Acta Carsologica 42:301–309, http://ojs.zrc-sazu.si/carsologica/article/view/669
Park MS, Takeda M (2009) Starvation induces apoptosis in the midgut nidi of Periplaneta americana: a histochemical and ultrastructural study. Cell Tissue Res 335(3):631–638
Park MS, Takeda M (2014) Cloning of PaAtg8 and roles of autophagy in adaptation to starvation with respect to the fat body and midgut of the American cockroach, Periplaneta americana. Cell Tissue Res 356(2):405–416
Pehani S, Virant-Doberlet M, Jeram S (1997) The life cycle of the cave cricket Troglophilus neglectus Krauss with a note on T. cavicola Kollar (Orthoptera: Rhaphidophoridae). Entomologiste 116:224–238
Polver PD, Sacchi L, Grigolo A, Laudani U (1986) Fine structure of the fat body and its bacteroids in Blattella germanica (Blattodea). Acta Zool 67:63–71
Pontes EG, Leite P, Majerowicz D, Atella GC, Gondim KC (2008) Dynamics of lipid accumulation by the fat body of Rhodnius prolixus: the involvement of lipophorin binding sites. J Insect Physiol 54:790–797
Rideout HJ, Lang-Rollin I, Stefanis L (2004) Involvement of macroautophagy in the dissolution of neuronal inclusions. Int J Biochem Cell Biol 36:2551–2562
Romanelli D, Casati B, Franzetti E, Tettamanti G (2014) A molecular view of autophagy in lepidoptera. Review article. Hindawi Publishing Corporation, BioMed Research International. Article ID 902315: 11. doi: 10.1155/2014/902315
Rost-Roszkowska MM, Poprawa I, Kaczmarek L (2011) Autophagy as the cell survival in response to a microsporidian infection of the midgut epithelium of Isohypsibius granulifer granulifer (Eutardigrada:Hypsibiidae). Acta Zool. doi:10.1111/j.1463-6395.2011.00552.x
Rost-Roszkowska MM, Vilimova J, Sosinka A, Skudlik J, Franzetti E (2012) The role of autophagy in the midgut epithelium of Eubranchipus grubii (Crustacea, Branchiopoda, Anostraca). Arthropod Struct Dev 41(3):271–279
Rost-Roszkowska MM, Chajec Ł, Vilimova J, Tajovský K, Kszuk-Jendrysik M (2015) Does autophagy in the midgut epithelium of centipedes depend on the day/night cycle? Micron 68:130–139
Santos DE, Azavedo DO, Campos LAO, Zanuncio JC, Serrão (2015) Melipona quadrifasciata (Hymenoptera: Apidae) fat body persists through metamorphosis with a few apoptotic cells and an increased autophagy. Protoplasma 252:619–627
Schmid D, Münz C (2007) Innate and adaptive immunity through autophagy. Immunity 26:11–21
Scott RC, Schuldiner O, Neufeld TP (2004) Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell 7(2):167–178
Sumithra P, Britto CP, Krishnan M (2010) Modes of cell death in the pupal perivisceral fat body tissue of the silkworm Bombyx mori L. Cell Tissue Res 339(2):349–358
Suzuki SW, Onodera J, Ohsumi Y (2011) Starvation induced cell death in autophagy-defective yeast mutants is caused by mitochondria dysfunction. PLoS ONE 6(2), e17412. doi:10.1371/journal.pone.0017412
Tashiro Y, Shimadzu T, Matsuura S (1976) Lysosomes and related structures in the posterior silk gland cells of Bombyx mori. I. In late larval stadium. Cell Struct Funct 1(3):205–222
Tettamanti G, Saló E, González-Estévez C, Felix DA, Grimaldi A, de Eguileor M (2008) Autophagy in invertebrates: insights into development, regeneration and body remodeling. Curr Pharm Des 14(2):116–125
Tsuchida K, Wells MA (1988) Digestion, absorption, transport and storage of fat during the last larval stadium of Manduca sexta. Changes in the role of lipophorin in the delivery of dietary lipid to the fat body. Insect Biochem 18:263–268
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12(9):814–822
Yorimitsu T, Klionsky DJ (2005) Autophagy: molecular machinery for self-eating. Cell Death Differ 12:1542–1552
Zara FJ, Caetano FH (2004) Ultramorphology and histochemistry of fat body cells from last Instar larval of the Pachycondyla (=Neoponera) villosa (Fabricius) (Formicidae: Ponerinae). Braz J Biol 64:725–735
Acknowledgments
We thank Elisabeth Bock, Rudi Schmied (Medical University Graz), and Rudi Mlakar (Faculty of Medicine, University of Maribor) for their excellent technical assistance, Franc Janžekovič for the statistical analysis of the data, and Michelle Gadpaille for the linguistic improvements to the manuscript. We are grateful to two anonymous reviewers for their insightful comments and suggestions. This study was partly supported by the Biodiversity Research Programme, Grant P1-0078.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Lucy M Collinson
Rights and permissions
About this article
Cite this article
Lipovšek, S., Novak, T. Autophagy in the fat body cells of the cave cricket Troglophilus neglectus Krauss, 1878 (Rhaphidophoridae, Saltatoria) during overwintering. Protoplasma 253, 457–466 (2016). https://doi.org/10.1007/s00709-015-0824-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0824-3