Abstract
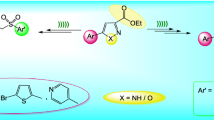
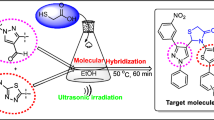
An environmentally benign, simple, efficient, and convenient route is described for the synthesis of novel pyrazolo[1,5-a]pyrimidine derivatives under ultrasound irradiation assisted by KHSO4 in aqueous medium. 3-(4-Methoxyphenyl)-3-oxopropanenitrile reacted with hydrazine hydrate in refluxing ethanol to give 5-(4-methoxyphenyl)-1H-pyrazol-3-amine. Condensation of 3-aminopyrazoles with formylated active proton compounds furnished pyrazolopyrimidines in high to excellent yield. The chemical structure and regioselectivity of the synthesized compounds were confirmed by IR, 1H NMR, 13C NMR, and mass spectral data. X-ray crystallographic study of a selected compound was performed. Furthermore, these synthesized compounds were screened for their anti-inflammatory and anti-cancer activity and the results were promising. The major advantages of this protocol afford high yields, operational simplicity, short reaction times, and devoid of harsh reaction conditions.
Graphical abstract








Similar content being viewed by others
References
Elnagdi MH, Elmoghayar MRH, Elgemeie GEH (1987) Adv Heterocycl Chem 41:319
Regan AC (2008) Pyrazolo[1,5-c]pyrimidine (73). In: Katritzky AR, Ramsden CA, Scriven EFV, Taylor RJK (eds) Comprehensive heterocyclic chemistry III, vol 11. Elsevier, Oxford, p 577
Youssef S (1997) Monatsh Chem 128:493
Kandeel ZE, Hafez EA, Sleim MA, Abdelatif FM, Elnagdi MH (1995) Heteroat Chem 6:305
Stepaniuk OO, Matviienko VO, Kondratov IS, Vitruk IV, Tolmachev AO (2012) Synthesis 45:925
Kalita U, Kaping S, Nellanant J, Helissey P, Vishwakarma JN (2014) Heteroletters 4:137
Devi AS, Kaping S, Vishwakarma JN (2015) Mol Divers 19:759
Kaping S, Boiss I, Singha LI, Helissey P, Vishwakarma JN (2015) Mol Divers. doi:10.1007/s11030-015-9639-6
Petrov AA, Emelina EE, Selivanov SI (2008) Russ J Org Chem 44:263
Quiroga J, Mejia D, Insuasty B, Abonia R, Nogueras M, Sanchez A, Cobo J (2002) J Heterocycl Chem 39:51
Aggarwal R, Sumran G, Garg N, Aggarwal A (2011) Eur J Med Chem 46:3038
Behbehani H, Ibrahim HM, Makhseed S, Mahmoud H (2011) Eur J Med Chem 46:1813
Compton DR, Carlson KE, Katzenellenbogen JA (2004) J Med Chem 17:5872
Mokhtara M, Saleha TS, Basahel SN (2012) J Mol Catal A 353–354:122
Shaaban MR, Saleh TS, Farag AM (2007) Heterocycles 71:1765
Behbehani H, Ibrahim HM, Makhseed S (2010) Arkivoc 2010(ii):267
Baluja S, Kachhadia N, Solanki A (2013) Open J Org Chem 1:1
Nagargoje D, Mandhane P, Shingote S, Badadhe P, Gill C (2012) Ultrason Sonochem 19:94
Singh BS, Lobo HR, Pinjari DV, Jarag KJ, Pandit AB, Shankarling GS (2013) Ultrason Sonochem 20:287
Jarag KJ, Pinjari DV, Pandit AB, Shankarling GS (2011) Ultrason Sonochem 18:617
Banitaba SH, Safari J, Khalili SD (2013) Ultrason Sonochem 20:401
Bazgir A, Ahadi S, Ghahremanzadeh R, Khavasi HR, Mirzaei P (2010) Ultrason Sonochem 17:447
Khosropour AR (2008) Ultrason Sonochem 15:659
Gopalsamy A, Ciszewski G, Shi M, Berger D, Hu Y, Lee F, Feldberg L, Frommer E, Kim S, Collins K, Wojciechowicz, Mallon R (2009) Bioorg Med Chem Lett 19:6890
Chanda K, Dutta MC, Karim E, Vishwakarma JN (2004) J Indian Chem Soc 81:791
Radl S, Blahovcova M, Tkadlecová M, Havlicek J (2010) Heterocycles 80:1359
Alam A, Imliwati L, Rapthap C, Singh V (2001) Indian J Exp Biol 39:201
Ferrari M, Fomasiero MC, Isetta AM (1990) J Immunol Methods 131:165
Singh A, Malhotra S, Subban R (2008) Int J Integr Biol 3:57
Khan FH (2009) The elements of Immunology. Pearson education, India
Ahmad F, Khan R, Rasheed S (1992) J Islam Acad Sci 5:111
Kang OH, Chae HS, Oh YC, Choi JG, Lee YS, Jang HJ, Kim JH, Kim YC, Sohn DH, Park H, Kwon DY (2008) Am J Chin Med 36:913
Mantovani NV, Germano G, Marchesi F, Locatelli M, Biswas SK (2011) Eur J Immunol 41:2522
Freshney RI (2010) Culture of animal cells: a manual of basic technique and specialized applications, 6th edn. Wiley, New Jersey, p 373
Lai SC, Peng WH, Huang SC, Ho YL, Huang JH, Lai Z, Chang Y (2009) Am J Chin Med 37:573
Houwen B (2000) Lab Hematol 6:1
Acknowledgments
Authors wish to thank Rev. Fr. Dr. Stephen Mavely, Vice Chancellor, Assam Don Bosco University for providing infrastructure for the execution of this work. Authors also wish to express their gratitude to IIT, Guwahati, Tezpur University, Tezpur, SAIF-NEHU, Shillong and SAIF-CDRI, Lucknow. Our thanks are also due to the Department of Biotechnology (DBT), Government of India for a research grant. SK thanks NER-BPMC-DBT, New Delhi for a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaping, S., Kalita, U., Sunn, M. et al. A facile, regioselective synthesis of pyrazolo[1, 5-a]pyrimidine analogs in the presence of KHSO4 in aqueous media assisted by ultrasound and their anti-inflammatory and anti-cancer activities. Monatsh Chem 147, 1257–1276 (2016). https://doi.org/10.1007/s00706-015-1638-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1638-x